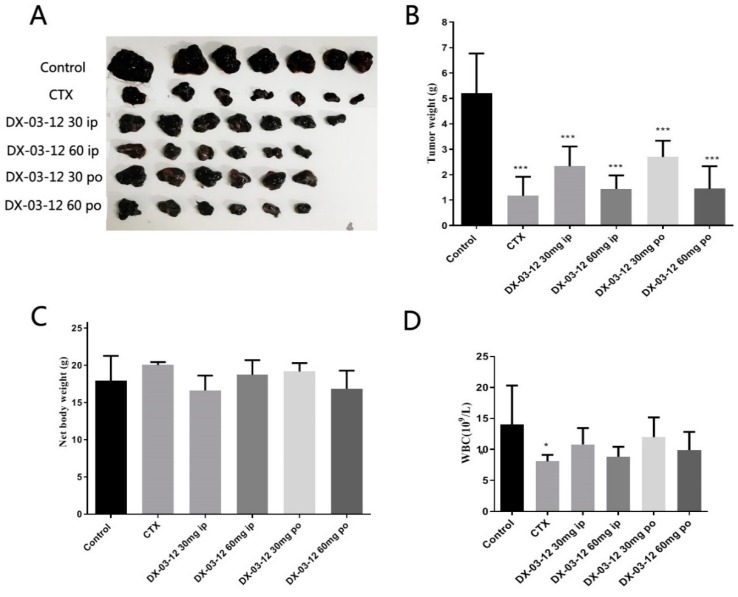
Figure 4.
In vivo anti-tumor activity of DX-03-12 in B16F10 xenograft mice. (A,B) Tumors from each group after 19 days of treatment. Mice bearing B16F10 xenografts were dosed orally or i.p. with vehicle (0.5% methylcellulose), CTX (Cyclophosphamide, orally, 60 mg/kg twice a week), DX-03-12 (i.p., 30 mg/kg/day), DX-03-12 (i.p., 60 mg/kg/day), DX-03-12 (orally, 30 mg/kg/day), or DX-03-12 (orally, 60 mg/kg/day). Results are expressed as the mean ± SD (n = 5–7 for each group). * p < 0.05 and *** p < 0.001 versus vehicle. (C) The body weight from each group after the treatment. There is no obvious body weight difference among all of the groups. (D) The white blood cells detected in the blood of all groups of animals. There is also no obvious WBC difference among all of the groups.