Figure 1.
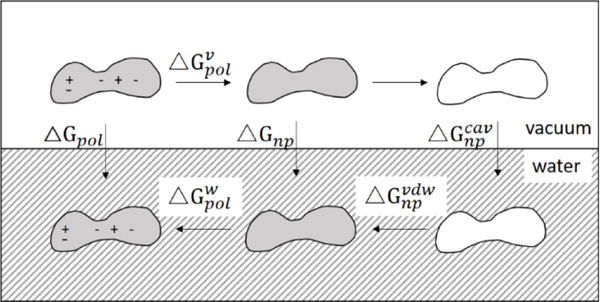
Thermodynamic cycle of the solvation process. Solvation free energy (△Gsol) is decomposed into polar (△Gpol) and nonpolar(△Gnp) contributions. The steps involve uncharging the solute in vacuum , removing the solute-solvent interaction in vacuum (no free energy change), creating a solute cavity (), establishing uncharged solute-solvent interaction in solvent (), and charging the solute in solvent (). The figure is adapted from Levy et al.22
