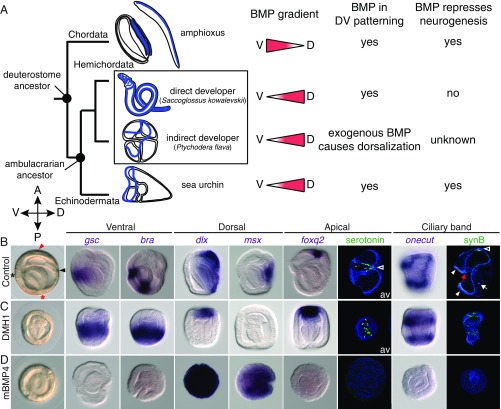
Fig. 1.
BMP controls DV patterning and represses neurogenesis in the indirect-developing hemichordate. (A) Deuterostome phylogeny shows nervous systems (blue) of Chordata (amphioxus embryo and adult as representatives), direct- and indirect-developing hemichordates, and the indirect-developing sea urchin embryo. Directions of BMP gradients (red triangles) along the dorsal (D) and ventral (V) axes and their roles in DV patterning and neural development are indicated for each lineage. (B–D) Controls cultured with DMSO or 125 ng/mL BSA (B), and embryos treated with 2 μM DMH1 (C) or 125 ng/mL mBMP4 (D). The treatments were performed after fertilization, and the embryos were fixed at the gastrula stage for in situ hybridization with ventral, dorsal, apical, and ciliary band marker genes. Except the tornaria larvae stained for serotonin, which are viewed from the apical surface (av), all other images are side views, with the directions of the anterior (A), posterior (P), dorsal (D), and ventral (V) axes indicated. Positions of the mouth (black asterisk), the anus (red asterisk), the hydropore opening from the dorsally extended mesoderm (black arrowhead), and the apical region (red arrowhead) are indicated in the control gastrula. The nervous system of tornaria larva includes serotonergic neurons in the apical ectoderm (empty arrowheads) and the neurons distributed along the ciliary band (white arrowheads). Some neuronal cells are also found in the pharynx (red arrow) and near the sphincter between the midgut and hindgut (white arrow). Neurons in the apical ectoderm and the ciliary band were visualized at the tornaria stage by immunostaining with anti-serotonin and anti-synB antibodies, respectively. The data represent the phenotypes of most samples (>95%).