Abstract
Recently, microRNA (miR)-628 was identified as a potential biomarker for several types of cancer, including prostate cancer (PCa). The aim of the present study was to investigate miR-628 expression and its underlying mechanism in PCa cell proliferation and invasion and the fibroblast growth factor receptor 2 (FGFR2) signaling pathway. The serum expression levels of miR-628, prostate-specific antigen, fibroblast growth factor 1, and FGFR2 were examined in patients with PCa. The relative expression levels of miR-628 and FGFR2 were determined by reverse transcription-quantitative polymerase chain reaction in PCa cells following transfection with miR-628-5p mimic or inhibitor. In addition, the protein expression level of FGFR2 was examined by western blot analysis following transfection with miR-628-5p mimic or inhibitor. Following bioinformatics analysis, dual-luciferase reporter assay was used to confirm the direct interaction between miR-628 and FGFR2. The current study demonstrated that the protein expression level of FGFR2 decreased following transfection with miR-628-5p mimic and increased following transfection with miR-628-5p inhibitor. Similarly, the proliferation and invasion of PCa cells were significantly enhanced following transfection with miR-628-5p inhibitor. By contrast, the proliferation and invasion of PCa cells were significantly inhibited following transfection with miR-628 mimic. Therefore, downregulating the expression level of miR-628 may increase the expression level of FGF in PCa, thereby promoting tumor proliferation and invasion. In conclusion, the FGF signaling pathway may be involved in promoting PCa cell proliferation and invasion. miR-628 may be a potential therapeutic target for patients with PCa.
Keywords: microRNA-628, prostate cancer, fibroblast growth factor signaling pathway, proliferation, migration
Introduction
Prostate cancer (PCa) is the most common malignancy and the leading cause of cancer mortality among men in the USA and Europe (1,2). The male population with a median age >60 years is more susceptible to PCa (3,4). In total, there are >1 million newly diagnosed cases of PCa identified each year and ~300,000 cases succumb to the disease (5). The incidence and mortality rates of PCa vary due to genetic variation and environmental changes between different age and ethnic groups (3,5–7). Exercise, obesity, smoking, vitamins, micronutrients, metformin, statins and other medications are all considered to be critical factors, which can affect the incidence and mortality rates of PCa (5,6). In addition, the incidence rate increases significantly in urban areas of China, South Korea and other countries (1,3,6).
Testosterone serves a key role in the development, growth and maintenance of the prostate (8,9) and abnormal prostate cell proliferation and differentiation caused by hormonal regulation may be involved in the initiation of hypertrophy, hyperplasia and malignancy (8,9). Many growth factors, including epidermal growth factor, fibroblast growth factor (FGF), insulin-like growth factor, transforming growth factor β1 and keratinocyte growth factor are also involved (8,10). FGFs can activate FGF receptors (FGFRs), which can lead to wound repair and neovascularization, as well as tumor growth and differentiation, thereby making FGF a potential therapeutic target in the development of novel anti-cancer treatments (11,12).
Early diagnosis of PCa is key to reducing morbidity and mortality (8). The main treatment options for early-stage PCa involve monitoring the prostate, whereas late-stage PCa requires radiation, surgery and other treatments, such as systemic chemotherapies or targeted therapies (8). Radical prostatectomy followed by radiotherapy in combination with hormonal treatment is widely used to treat patients with recurrent PCa (8,13). Furthermore, treatment outcome varies dramatically as clinical heterogeneity is another feature associated with PCa (8,13).
microRNAs (miRNAs or miRs) are single-stranded small RNAs, 18–23 nucleotides in length which can regulate gene expression through mRNA degradation or protein synthesis inhibition (14,15). miRNAs can regulate several physiological or pathological processes including cell proliferation, differentiation, stress and death (14,15). miRNAs have been identified in several types of cancer and can function as positive or negative regulators, depending on their target gene (16,17). miR-628, which is located at 15q21.3, was initially identified in acute myeloid leukemia and is regulated by interleukin-3, granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor (15). A previous study demonstrated that miR-628 is an endotoxin-responsive gene induced by lipopolysaccharide as well as a nuclear factor κB-dependent gene (18). miR-628 can regulate inflammatory responses by targeting a key adapter molecule downstream of toll-like receptors (TLRs) to suppress TLR signaling (18).
In addition, miR-628 mediates burn-induced skeletal muscle atrophy through regulation of the insulin receptor substrate 1/protein kinase B serine/threonine kinase 1/forkhead box protein O 3a signaling pathway (19). miR-628-3p is upregulated in patients with bone fractures and miR-628-3p can exert an inhibitory effect on osteogenesis via the suppression of runt related transcription factor 2 leading to atrophic non-union, a complication associated with bone fractures (20). Furthermore, miR-628-3p is involved in rifampin-mediated cytochrome P450 family 3 subfamily A member 4 (CYP3A4) induction as it is thought to directly target CYP3A4 (21). miR-628-5p has previously been demonstrated to decrease the stem-like cell percentage of epithelial ovarian cancer (EOC) cells by inducing their apoptosis as well as decreasing the tumorigenicity of EOC cells by targeting FGFR2 (11).
A previous study identified miR-628 as a potential non-invasive biomarker for PCa (14), however whether miR-628 serves a tumor suppressing role in PCa remains unknown. In addition, whether FGFR2 is a direct target of miR-628 in PCa remains unknown. Therefore, the aim of the present study was to investigate miR-628 expression and its underlying mechanism in PCa cell proliferation and invasion and the FGFR2 signaling pathway.
Materials and methods
PCa and healthy control patients
A total of 33 patients with PCa (males; age range, 55–89 years; average age, 70 years) were recruited in Wuhan Fourth Hospital, Puai Hospital (Wuhan, China) between January 2016 and December 2017 for inclusion in the current study. Patients were diagnosed following prostate biopsy or postoperative pathological examination. Patients with PCa with a Gleason score range from 4–10 were included in the study. In total, 12 patients had a Gleason score of 4–6 (well differentiated), 9 patients had a Gleason score of 7 (medium differentiated) and 12 patients had a Gleason score of 8–10 (low differentiated). A summary of the cancer staging and Gleason score for patients with PCa is presented in Table I. In addition, 26 healthy control patients (age range, 62–82 years; average age, 79 years) were recruited following admittance to the hospital physical examination center of Wuhan Fourth Hospital, Puai Hospital (Wuhan, China) for a health check-up between January 2016 and December 2017. Blood pressure, urine routine test, and liver and kidney function were all within the normal range. Patients with other diseases affecting heart, lungs or other vital organs, such as benign prostatic hyperplasia and other prostate-associated diseases were excluded. The current study was approved by the Ethics Committee of Wuhan Fourth Hospital, Puai Hospital (Wuhan, China) and written informed consent was obtained from all participants.
Table I.
Serum levels of PSA, FGF1 and FGFR2 in patients with PCa.
| Group | n | PSA (ng/l) | FGF1 (pg/ml) | FGFR2 (pg/ml) |
|---|---|---|---|---|
| Control | 26 | 1.03±0.62 | 203.72±25.27 | 64.14±11.11 |
| PCa | 33 | 21.65±12.18 | 304.02±45.79 | 88.26±10.09 |
| Gleason score | ||||
| 4–6 | 12 | 13.42±5.69 | 304.93±57.13 | 88.60±12.92 |
| 7 | 9 | 20.87±12.86 | 318.36±29.26 | 88.40±8.19 |
| 8–10 | 12 | 30.46±10.97 | 292.36±43.54 | 87.03±9.24 |
| Clinical stage | ||||
| T1 | 4 | 8.09±1.94 | 314.42±60.76 | 89.125±12.17 |
| T2 | 6 | 15.15±5.51 | 304.03±54.08 | 88.65±14.13 |
| T3 | 15 | 20.34±10.35 | 308.78±43.85 | 88.19±8.56 |
| T4 | 8 | 35.76±8.27 | 289.89±41.58 | 86.49±10.86 |
PSA, prostate-specific antigen; FGF1, fibroblast growth factor 1; FGFR2, fibroblast growth factor receptor 2; PCa, prostate cancer.
Specimen collection
Venous blood samples (4 ml) were collected from all participants (PCa and control) in the fasting state prior to any treatment, which included medication, hormonal treatment, surgery, as well as rectal examination, prostate massage and puncture within 1 week before blood extraction. Blood was centrifuged at 1,200 × g at room temperature for 12 min to collect serum. Serum prostate-specific antigen was measured immediately using an ADVID Centaur CP analyzer (Siemens Healthineers, Erlangen, Germany). The remaining serum was stored at −20°C and used to determine serum expression levels of miR-628, FGF-1 and FGFR2 using an IMMULITE®1000 immunoassay system (Siemens Healthineers).
Cell culture
Human prostatic adenocarcinoma cell line LNCaP (ATCC® CRL-1740™) was purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium (ATCC) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin, and maintained at 37°C in an atmosphere containing 5% CO2.
Cell transfection
MiR-628-5p mimic (5-AUG CUG ACA UAU UUA CUA GAGG-3; cat. no. MCH03275) and negative control miRNA (cat. no. MCH00000), as well as miR-628-5p inhibitor (cat. no. MIH03275) or negative control inhibitor (cat. no. MIH00000) was purchased from Applied Biological Materials Inc., (Richmond, BC, Canada). Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) was used to transfect 35 nM miR-628-5p mimic and negative control miRNA (negative control), as well as 35 nM miR-628-5p inhibitor or negative control inhibitor (negative control) into 105 cells. Untransfected cells were considered control cells. Subsequent experiments were performed at 24 h post-transfection.
MTT cytotoxicity assays
LNCaP cells were seeded into 96-well plates (3×104 cells in 0.1 ml culture medium per well) and transfected with miR-628-5p mimic (cat. no. MCH03275), miR-628-5p inhibitor (cat. no. MIH03275; both Applied Biological Materials Inc., Richmond, BC, Canada) or control. Following 12-h incubation, 10 µl MTT solution (5 mg/ml) was added and cells were incubated for a further 2 h at 37°C. Following incubation, cell culture medium was removed and dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to dissolve the purple formazan crystals. Optical density was measured at 570 nm using a luminometer microplate reader (Berthold Technologies GmbH, Bad Wildbad, Germany).
Cell invasion assay
Transwell inserts (8 µm) were used. Upper chambers were pre-coated with 40 ml Matrigel® (BD Biosciences, Franklin Lakes, NJ, CA, USA) dissolved in 20% culture medium and incubated overnight at 37°C. In total, 5×104 cells collected at 24 h after transfection were suspended in 200 ml serum-free RPMI-1640 medium and were plated in the upper chamber. RPMI-1640 medium supplemented with 20% FBS (Gibco; Thermo Fisher Scientific, Inc.) was plated in the lower chamber. Following incubation for 36 h at 37°C, cells were stained with 0.1% crystal violet at room temperature for 20 min. Stained cells were observed under an optical microscope (magnification, ×40).
ELISA
Prostate-specific antigen (PSA), fibroblast growth factor (FGF)1 and FGFR2 in plasma were detected by performing ELISA using human Prostate Specific Antigen ELISA Kit (cat. no. ab188388; Abcam), Human FGF1 ELISA Kit (cat. no. ab219636; Abcam) and FGFR2 ELISA kit (cat. no. MBS921985; MyBioSource; Thermo Fisher Scientific, Inc.) respectively.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from LNCaP cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (≤500 ng) was reverse transcribed into cDNA using the High Capacity RNA-to-cDNA kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). To examine the mRNA expression level of FGFR2, qPCR was performed using the SYBR™ Green Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). To examine the expression level of miR-628 expression, qPCR was performed using the TaqMan probe kit (Roche Applied Science, Penzberg, Germany). qPCR was performed using an ABI 7900 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following primer pairs were used for qPCR: FGFR2 forward, 5′-CGCTGGTGAGGATAACAACACG-3′ and reverse, 5′-TGGAAGTTCATACTCGGAGACCC-3′. GAPDH forward, 5′-GTCTCCTCTGACTTCAA-3′ and reverse, 5′-ACCACCCTGTTGCTGTA-3′. miR-628 forward primer: 5′-GTCGTATCCAGTGCAGTTATATCC-3′. miR-628 reverse primer and U6 primers were included in the kit. The thermocycling conditions for qPCR were: Initial denaturation at 95°C for 10 sec; 40 cycles of 95°C for 10 sec and 60°C for 60 sec. FGFR2 and miR-628 expression was quantified using the 2−ΔΔCq method (15) and normalized to the internal reference gene GAPDH or U6 small non-coding RNA, respectively.
Western blot analysis
Total protein was extracted from LNCaP cells using the M-PER™ Mammalian Protein Extraction reagent (Pierce; Thermo Fisher Scientific, Inc.). Protein concentration was determined by BCA assay. An equal volume of protein was mixed with an equal volume of 2X SDS loading buffer and boiled for 5 min. Proteins (30 µg per lane) were separated via SDS-PAGE on a 10% gel and transferred onto polyvinylidene difluoride membranes (Zhongshan Jinqiao Biology & Technology Co., Ltd., Beijing, China). Membranes were blocked with 5% skimmed milk at room temperature for 2 h. The membranes were incubated with primary antibodies against FGFR2 (1:800; cat. no. ab58201; Abcam, Cambridge, MA, USA) and GAPDH (1:800; cat. no. sc-47724; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 2–8°C. Following primary incubation, membranes were incubated with horseradish peroxidase-labeled goat anti-mouse immunoglobulin G secondary antibody (1:1,200; cat. no. ZB2305; Zhongshan Jinqiao Biology & Technology Co., Ltd.) at room temperature for 3 h. Protein bands were visualized using a Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare Life Sciences, Shanghai, China).
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org/vert_72/) and miRDB (http://mirdb.org/) bioinformatics software was used to identify the 3′ untranslated region (UTR) of FGFR2 as a putative target of miR-628. The wild-type (wt) and mutant (mut) 3′UTR of FGFR2, which contains a sequence targeting miR-628, were cloned into the psi-CHECK vector (Promega Corporation, Madison, WI, USA). Cells were co-transfected with either miR-628-5p mimic (or miR-628-5p inhibitor) and psiFGFR2-wt (or psiFGFR2-mut) using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). Control miRNA or control inhibitor were used as controls. Following 48-h transfection, cells were harvested and luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega Corporation) using a luminometer (Berthold Technologies GmbH). Firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Data were expressed as mean ± standard deviation. All statistical analyses were performed using Sigmaplot v.11 (Systat Software Inc., Chicago, IL, USA) and SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Welch's t-test was used to analyze differences between two groups, while one-way analysis of variance and Tukey's post hoc test were used to analyze comparisons among multiple groups. Correlation analysis between the serum expression level of miR-628 and FGF-1 or FGFR2 was analyzed by Pearson's correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-628 expression is decreased in the serum of patients with PCa
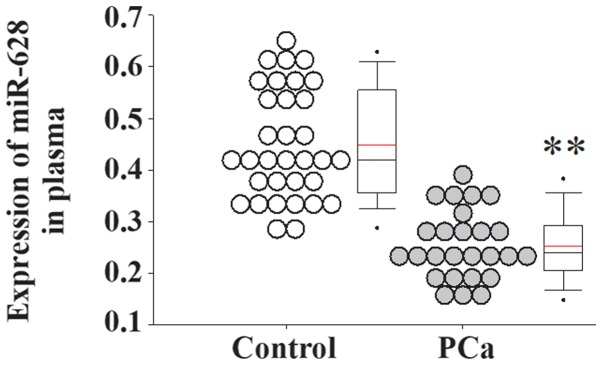
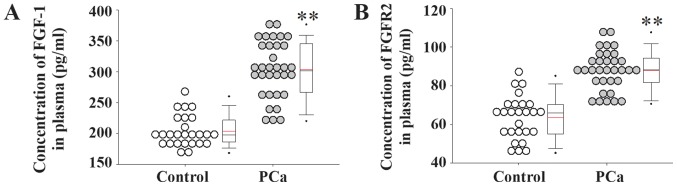
Serum samples from patients with PCa and healthy controls were collected and used for gene expression analyses. The serum expression level of miR-628 was significantly decreased in patients with PCa compared with healthy controls (Fig. 1). In addition, the serum expression levels of FGF1 and its receptor FGFR2 were significantly increased in patients with PCa compared with healthy controls (Fig. 2).
Figure 1.
miR-628 expression in patients with PCa. Serum expression levels of miR-628 in patients with PCa and healthy controls were determined using an immunoassay system. *P<0.01 vs. control. miR, microRNA; PCa, prostate cancer.
Figure 2.
Expression levels of FGF1 and FGFR2 in patients with PCa. Serum expression levels of (A) FGF1 and (B) FGFR2 in patients with PCa and healthy controls. **P<0.01 vs. control. FGF1, fibroblast growth factor 1; FGFR2, fibroblast growth factor receptor 2; PCa, prostate cancer.
miR-628 expression negatively correlates with FGFR2
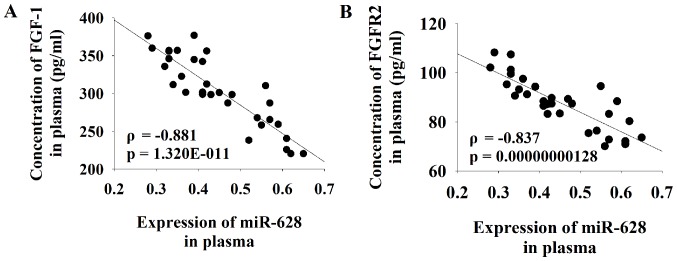
Serum levels of PSA were increased in patients with PCa compared with healthy controls (Table I). Pearson correlation coefficient analysis was performed to determine the association between the serum expression level of miR-628 and FGF1 or FGFR2. The results of the current study demonstrated that there was a significant negative correlation between the serum level of miR-628 and both FGF1 and FGFR2 in patients with PCa (Fig. 3).
Figure 3.
Correlation analysis between the serum expression levels of miR-628 and FGF-1 or FGFR2 in patients with PCa. Pearson correlation coefficient analyses were performed to determine the association between the serum expression level of miR-628 and (A) FGF1 or (B) FGFR2. miR, microRNA; FGF1, fibroblast growth factor 1; FGFR2, fibroblast growth factor receptor 2; PCa, prostate cancer.
miR-628 regulates FGFR2 signaling
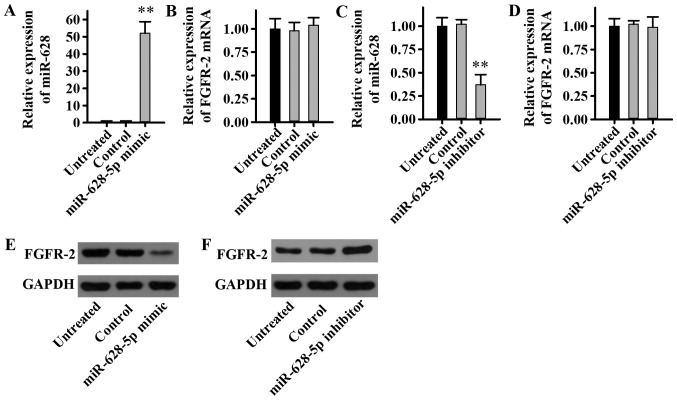
To determine the effect of miR-628 on the expression level of FGFR2, LNCaP cells were transfected with miR-628-5p mimic, inhibitor or negative controls, and miR-628 and FGFR2 expression levels were examined. The current study demonstrated that the miR-628-5p mimic significantly increased miR-628 expression (Fig. 4A), whereas the miR-628-5p mimic had no significant effect on the mRNA expression level of FGFR2 in LNCaP cells (Fig. 4B). In addition, the miR-628-5p inhibitor significantly decreased miR-628 expression (Fig. 4C), while the miR-628-5p inhibitor had no significant effect on the mRNA expression level of FGFR2 in LNCaP cells (Fig. 4D). Although there was no effect on the FGFR2 mRNA expression level, the FGFR2 protein expression level was revealed to be potentially regulated by miR-628. The protein expression level of FGFR2 decreased markedly following transfection with miR-628-5p mimic (Fig. 4E), while the protein expression level of FGFR2 increased following transfection with miR-628-5p inhibitor (Fig. 4F). Taken together, these results suggest that miR-628 can inhibit FGFR2 protein expression in PCa cell lines without affecting transcriptional levels and therefore miR-628 may regulate FGFR2 expression.
Figure 4.
Effect of miR-628 on the expression level of FGFR2 in PCa cells. The relative expression level of (A) miR-628 and (B) FGFR2 was determined in the LNCaP cell line following transfection with miR-628-5p mimic. The relative expression level of (C) miR-628 and (D) FGFR2 was determined in the LNCaP cell line following transfection with miR-628-5p inhibitor. The protein expression level of FGFR2 was determined by western blot analysis in LNCaP cells following transfection with (E) miR-628-5p mimic or (F) miR-628-5p inhibitor. **P<0.01 vs. control. miR, microRNA; FGFR2, fibroblast growth factor receptor 2; PCa, prostate cancer; LNCaP, human prostatic adenocarcinoma cell line.
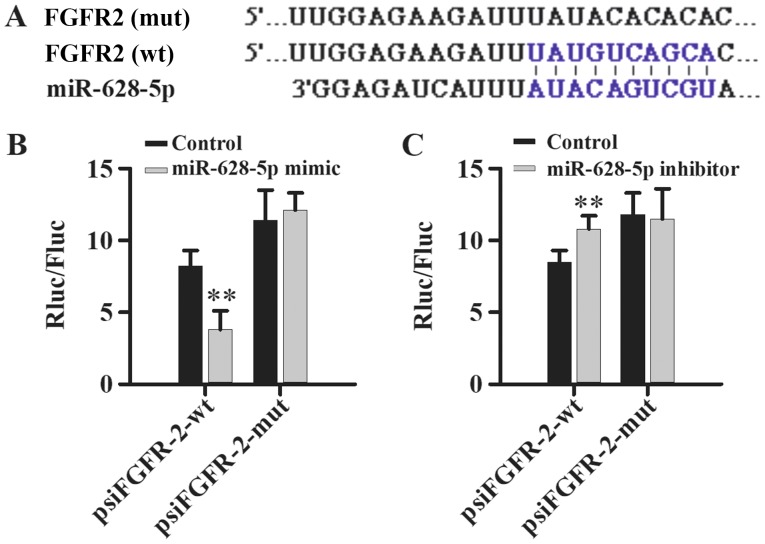
miR-628 directly targets FGFR2
TargetScan and miRDB bioinformatics software was used to identify the 3′UTR of FGFR2 as a putative target of miR-628 (Fig. 5A) and dual-luciferase reporter assays were performed to validate the direct interaction between miR-628 and FGFR2. Luciferase activity was significantly decreased following co-transfection with miR-628-5p mimic and psiFGFR2-wt compared with psiFGFR2-mut, which had no significant effect on luciferase activity (Fig. 5B). In addition, luciferase activity was significantly increased following co-transfection with miR-628-5p inhibitor and psiFGFR2-wt compared with psiFGFR2-mut, which had no effect on luciferase activity (Fig. 5C). Taken together, these results suggest that miR-628 directly interacts with FGFR2.
Figure 5.
FGFR2 is a direct target of miR-628. (A) TargetScan and miRDB bioinformatics software was used to predict the potential binding site for miR-628-5p binding site in the wild-type and mutant 3′UTR of FGFR2. Luciferase activity of the wt or mut 3′UTR of FGFR2 following transfection with (B) miR-628 mimic or (C) miR-628 inhibitor. **P<0.01 vs. control. miR, microRNA; FGF1, fibroblast growth factor 1; FGFR2, fibroblast growth factor receptor 2; UTR, untranslated region; wt, wild-type; mut, mutant.
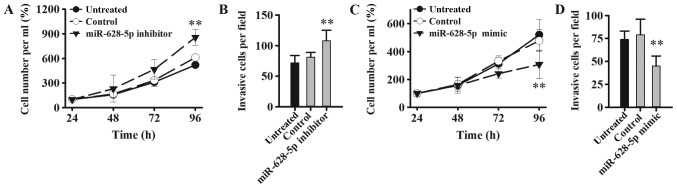
miR-628 regulates tumorigenesis via FGFR2 signaling pathway
The effect of miR-628 on PCa cell proliferation and invasion was examined in LNCaP cells following transfection with miR-628-5p mimic, inhibitor or negative controls. Cell proliferation and invasion was significantly increased in LNCaP cells following transfection with miR-628-5p inhibitor compared with the control group (Fig. 6A and B). By contrast, cell proliferation and invasion was significantly decreased in LNCaP cells following transfection with miR-628-5p mimic compared with the control group (Fig. 6C and D). FGF is known to promote the proliferation of tumor cells and the formation of tumor blood vessels (11) and therefore, downregulating miR-628 expression may increase the expression level of FGF in PCa and thereby promote tumor initiation, expansion and metastasis.
Figure 6.
miR-628 knockdown promotes the LNCaP cell proliferation and migration. (A) MTT assay was used to examine the cell proliferation of LNCaP cells following transfection with miR-628-5p inhibitor. (B) Cell invasion assay was analyzed via Transwell assay in LNCaP cells following transfection with miR-628-5p inhibitor. (C) MTT assay was used to examine cell proliferation of LNCaP cells following transfection with miR-628-5p mimic. (D) Cell invasion assay was analyzed via Transwell assay in LNCaP cells following transfection with miR-628-5p mimic. **P<0.01 vs. control. miR, microRNA; LNCaP, human prostatic adenocarcinoma cell line; PCa, prostate cancer.
Discussion
Several miRNAs, which include miR-21, miR-221 and miR-141, have been associated with PCa (20). Most of these miRNAs are involved in cell cycle, migration, metastasis, apoptosis, proliferation, angiogenesis and epithelial-to-mesenchymal transition (22). A previous study demonstrated that the serum expression level of miR-628 was significantly downregulated in African American and Caucasian American patients with PCa (14). Similarly, the current study confirmed that the serum expression level of miR-628 was significantly downregulated in Chinese patients with PCa. The FGFR2 signaling pathway is known to be involved in several types of cancer. A recent study demonstrated that miR-628 targeted and downregulated the expression of FGFR2 in ovarian cancer (11). In the current study, serum expression levels of FGF1, FGFR2 and PSA were examined in patients with PCa and controls. Serum FGF1 and FGFR2 were negatively correlated with the serum expression level of miR-628. In addition, dual-luciferase reporter assays revealed that FGFR2 is a direct target of miR-628. Furthermore, the current study demonstrated that miR-628 inhibited PCa cell proliferation and invasion via the FGFR2 signaling pathway.
The FGFR2 gene at human chromosome 10q26 encodes two isoforms, FGFR2b and FGFR2c, which function as FGFRs and are involved in tumorigenesis (23–25). Missense mutations, copy number variation, splicing and single nucleotide polymorphisms within intron 2 of the FGFR2 gene are associated with breast, bladder and gastric cancer whereby aberrant FGFR2 signaling activation induces proliferation and survival of tumor cells (23–25). FGFR2 alternative splicing serves a role in the progression of PCa from an androgen-sensitive to androgen-insensitive tumor (26–28). Although FGFR2 inhibitors are approved and used for cancer treatment (11), the current study identified miR-628, which may be used as a potential molecular target for the development of novel therapeutic treatment strategies for patients with PCa. In addition, targeting miR-628 may also be used to treat ovarian cancer as well as inflammation regulation (15,19).
Several technologies including human antibody, peptide mimetic, RNA aptamer, small interfering RNA and synthetic miRNA are emerging technologies, which can be applied and used as potential therapeutic treatment strategies in FGFR2-mediated cancer (24). In particular, these technologies may be used for the treatment of patients with PCa with low expression of miR-628 and upregulated FGFR2 signaling. Curcumin, for example, has a variety of pharmacological effects including anti-cancer effects and curcumin exerts its therapeutic effects through regulation of miRNA expression (17). miRNAs are thought to be potential targets for cancer therapy and as such there have been a number of studies regarding miRNA regulation via miRNA activators and inhibitors (16,29–32). However, developing novel delivery systems for miRNA treatment is key to its success as a potential therapy (31).
Further experiments are required to validate the findings of the current study using in vivo mouse models, such as examining tumorigenesis in a xenograft mouse model. In vivo mouse models for PCa could be used to study miR-628 expression and regulation of the FGFR2 signaling pathway, as well as testing newly designed miR-628 activators. However, a critical step will be to develop a delivery system for the efficient delivery of miRNAs into in vivo mouse models for PCa. The current study identified the FGFR2 signaling pathway as a potential therapeutic target, however, patients may become resistant to FGFR2-mediated therapy and alternative treatment may be required. Sequencing technology may be used to develop treatments combining FGFR2 inhibition and miR-628 upregulation, which may be a potentially effective approach for the treatment of patients with PCa.
In conclusion, the present study demonstrated that miR-628 directly targets FGFR2 and miR-628 can regulate tumorigenesis via the FGFR2 signaling pathway in PCa cells. Therefore, FGFR2 and miR-628 may be potential molecular targets for the development of novel and effective therapeutic treatment strategies for patients with PCa.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JC and YZ designed experiments. YZ and PH performed experiments. TZ analyzed data. YZ drafted this manuscript and all authors approved this manuscript.
Ethics approval and consent to participate
The current study was approved by the Ethics Committee of Wuhan Fourth Hospital, Puai Hospital (Wuhan, China). All patients and healthy volunteers provided written informed consent prior to their inclusion in the study.
Patient consent for publication
Patients provided consent for publication of data in this paper.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Han HH, Park JW, Na JC, Chung BH, Kim CS, Ko WJ. Epidemiology of prostate cancer in South Korea. Prostate Int. 2015;3:99–102. doi: 10.1016/j.prnil.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashir MN. Epidemiology of prostate cancer. Asian Pac J Cancer Prev. 2015;16:5137–5141. doi: 10.7314/APJCP.2015.16.13.5137. [DOI] [PubMed] [Google Scholar]
- 3.Ye D, Zhu Y. Epidemiology of prostate cancer in China: An overview and clinical implication. Zhonghua Wai Ke Za Zhi. 2015;53:249–252. (In Chinese) [PubMed] [Google Scholar]
- 4.Tao ZQ, Shi AM, Wang KX, Zhang WD. Epidemiology of prostate cancer: Current status. Eur Rev Med Pharmacol Sci. 2015;19:805–812. [PubMed] [Google Scholar]
- 5.Cooperberg MR, Chan JM. Epidemiology of prostate cancer. World J Urol. 2017;35:849. doi: 10.1007/s00345-017-2038-0. [DOI] [PubMed] [Google Scholar]
- 6.Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018;25:524–531. doi: 10.1111/iju.13593. [DOI] [PubMed] [Google Scholar]
- 7.Tourinho-Barbosa RR, Pompeo AC, Glina S. Prostate cancer in Brazil and Latin America: Epidemiology and screening. Int Braz J Urol. 2016;42:1081–1090. doi: 10.1590/s1677-5538.ibju.2015.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltran H, Antonarakis ES, Morris MJ, Attard G. Emerging molecular biomarkers in advanced prostate cancer: Translation to the clinic. Am Soc Clin Oncol Educ Book. 2016;35:131–141. doi: 10.14694/EDBK_159248. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Wang GM, Guo JM, Sun LA, Wang H. NGF/γ-IFN inhibits androgen-independent prostate cancer and reverses androgen receptor function through downregulation of FGFR2 and decrease in cancer stem cells. Stem Cells Dev. 2012;21:3372–3380. doi: 10.1089/scd.2012.0121. [DOI] [PubMed] [Google Scholar]
- 10.Adeola HA, Smith M, Kaestner L, Blackburn JM, Zerbini LF. Novel potential serological prostate cancer biomarkers using CT100+ cancer antigen microarray platform in a multi-cultural South African cohort. Oncotarget. 2016;7:13945–13964. doi: 10.18632/oncotarget.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Qian Z, Ma X, Lin X, You Y, Li Y, Chen T, Jiang H. MiR-628-5p decreases the tumorigenicity of epithelial ovarian cancer cells by targeting at FGFR2. Biochem Biophys Res Commun. 2018;495:2085–2091. doi: 10.1016/j.bbrc.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Schwertfeger KL. Fibroblast growth factors in development and cancer: Insights from the mammary and prostate glands. Curr Drug Targets. 2009;10:632–644. doi: 10.2174/138945009788680419. [DOI] [PubMed] [Google Scholar]
- 13.Pernar CH, Ebot EM, Wilson KM, Mucci L. The epidemiology of prostate cancer. Cold Spring Harb Perspect Med. 2018;8(pii):a030361. doi: 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava A, Goldberger H, Dimtchev A, Marian C, Soldin O, Li X, Collins SP, Suy S, Kumar D. Circulatory miR-628-5p is downregulated in prostate cancer patients. Tumour Biol. 2014;35:4867–4873. doi: 10.1007/s13277-014-1638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Favreau AJ, Sathyanarayana P. miR-590-5p, miR-219-5p: miR-15b and miR-628-5p are commonly regulated by IL-3, GM-CSF and G-CSF in acute myeloid leukemia. Leuk Res. 2012;36:334–341. doi: 10.1016/j.leukres.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryzgunova OE, Konoshenko MY, Laktionov PP. MicroRNA-guided gene expression in prostate cancer: Literature and database overview. J Gene Med. 2018;20:e3016. doi: 10.1002/jgm.3016. [DOI] [PubMed] [Google Scholar]
- 18.Mirzaei H, Masoudifar A, Sahebkar A, Zare N, Sadri Nahand J, Rashidi B, Mehrabian E, Mohammadi M, Mirzaei HR, Jaafari MR. MicroRNA: A novel target of curcumin in cancer therapy. J Cell Physiol. 2018;233:3004–3015. doi: 10.1002/jcp.26055. [DOI] [PubMed] [Google Scholar]
- 19.Jun H, Ying H, Daiwen C, Bing Y, Xiangbing M, Ping Z, Jie Y, Zhiqing H, Junqiu L. miR-628, a microRNA that is induced by Toll-like receptor stimulation, regulates porcine innate immune responses. Sci Rep. 2015;5:12226. doi: 10.1038/srep12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Li X, Liu L, Chai J, Haijun Z, Chu W, Yin H, Ma L, Duan H, Xiao M. miR-628Promotes burn-induced skeletal muscle atrophy via targeting IRS1. Int J Biol Sci. 2016;12:1213–1224. doi: 10.7150/ijbs.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Ji X, She F, Gao Y, Tang P. miR-628-3p regulates osteoblast differentiation by targeting RUNX2: Possible role in atrophic non-union. Int J Mol Med. 2017;39:279–286. doi: 10.3892/ijmm.2016.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosini P, Bonaccorsi L, Baldi E, Chiasserini C, Forti G, De Chiara G, Lucibello M, Mongiat M, Iozzo RV, Garaci E, et al. Androgen receptor expression induces FGF2, FGF-binding protein production and FGF2 release in prostate carcinoma cells: Role of FGF2 in growth, survival and androgen receptor down-modulation. Prostate. 2002;53:310–321. doi: 10.1002/pros.10164. [DOI] [PubMed] [Google Scholar]
- 23.Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5:1063–1071. [PubMed] [Google Scholar]
- 24.Ropiquet F, Huguenin S, Villette JM, Ronflé V, Le Brun G, Maitland NJ, Cussenot O, Fiet J, Berthon P. FGF7/KGF triggers cell transformation and invasion on immortalised human prostatic epithelial PNT1A cells. Int J Cancer. 1999;82:237–243. doi: 10.1002/(SICI)1097-0215(19990719)82:2<237::AID-IJC14>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Katoh Y, Katoh M. FGFR2-related pathogenesis and FGFR2-targeted therapeutics (Review) Int J Mol Med. 2009;23:307–311. doi: 10.3892/ijmm_00000132. [DOI] [PubMed] [Google Scholar]
- 26.Carstens RP, Eaton JV, Krigman HR, Walther PJ, Garcia-Blanco MA. Alternative splicing of fibroblast growth factor receptor 2 (FGF-R2) in human prostate cancer. Oncogene. 1997;15:3059–3065. doi: 10.1038/sj.onc.1201498. [DOI] [PubMed] [Google Scholar]
- 27.Muh SJ, Hovhannisyan RH, Carstens RP. A Non-sequence-specific double-stranded RNA structural element regulates splicing of two mutually exclusive exons of fibroblast growth factor receptor 2 (FGFR2) J Biol Chem. 2002;277:50143–50154. doi: 10.1074/jbc.M207409200. [DOI] [PubMed] [Google Scholar]
- 28.Naimi B, Latil A, Fournier G, Mangin P, Cussenot O, Berthon P. Down-regulation of (IIIb) and (IIIc) isoforms of fibroblast growth factor receptor 2 (FGFR2) is associated with malignant progression in human prostate. Prostate. 2002;52:245–252. doi: 10.1002/pros.10104. [DOI] [PubMed] [Google Scholar]
- 29.Catto JW, Alcaraz A, Bjartell AS, De Vere White R, Evans CP, Fussel S, Hamdy FC, Kallioniemi O, Mengual L, Schlomm T, Visakorpi T. MicroRNA in prostate, bladder, and kidney cancer: A systematic review. Eur Urol. 2011;59:671–681. doi: 10.1016/j.eururo.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Curtin CM, Castaño IM, O'Brien FJ. Scaffold-based microRNA therapies in regenerative medicine and cancer. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201700695. [DOI] [PubMed] [Google Scholar]
- 31.Hosseinahli N, Aghapour M, Duijf PHG, Baradaran B. Treating cancer with microRNA replacement therapy: A literature review. J Cell Physiol. 2018;233:5574–5588. doi: 10.1002/jcp.26514. [DOI] [PubMed] [Google Scholar]
- 32.Weidle UH, Birzele F, Kollmorgen G, Nopora A. Potential microRNA-related targets for therapeutic intervention with ovarian cancer metastasis. Cancer Genomics Proteomics. 2018;15:1–15. doi: 10.21873/cgp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.