Fig. 4.
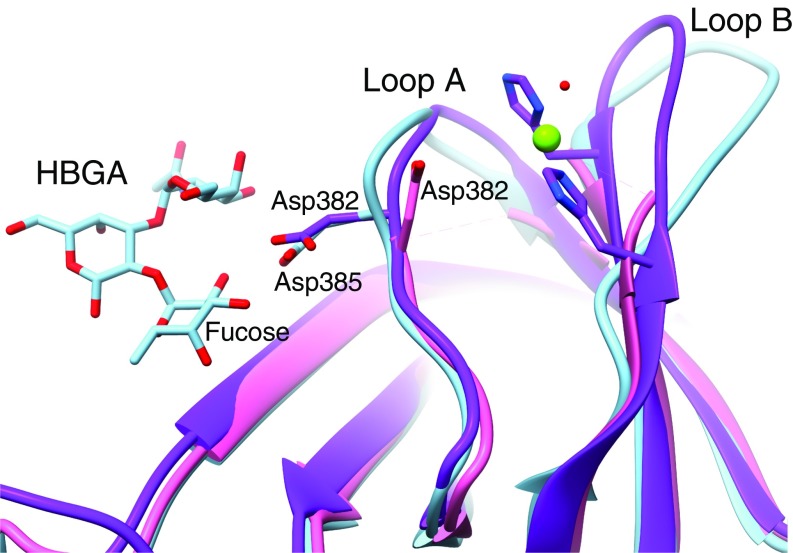
Histo-blood group antigen binding site in GII.2. The P-domain crystal structure of GII.10 with HBGA bound (PDB ID code 3PA1) was superimposed on the partial P-domain crystal structure of GII.2 (PDB ID code 4RPB) and our GII.2 full-subunit cryo-EM structure without HBGA bound to show the expected positioning of the HBGA binding site of GII.2. In HBGA-binding strains such as GII.10 (light blue), a conserved residue (Asp385) binds the fucose moiety of HBGAs. In the P-domain crystal structure of GII.2 (pink), the side chain of the equivalent residue (Asp382) was turned ∼180° away from the fucose binding site, and the hypervariable loop A bearing Asp382 and loop B were not observed. In our cryo-EM structures (purple), the hypervariable loops A and B are resolved with the Asp382 side chain pointed correctly toward the fucose binding site. A Zn2+ metal ion (green sphere) is bound between loops A and B immediately adjacent to the fucose binding site that may play a role as a coligand that enhances HBGA binding of the GII.2 strain.