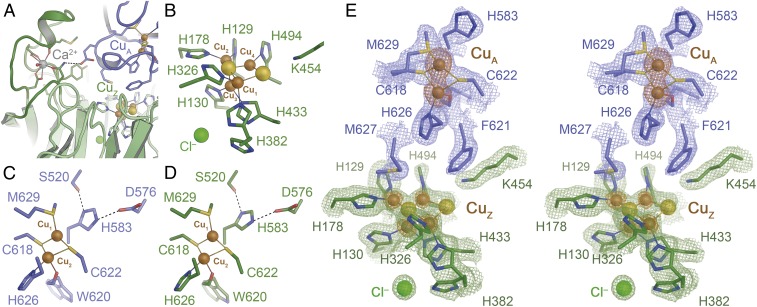
Fig. 3.
Three-dimensional structure of rNosZ. The recombinant protein fully retained the homodimer structure of all known NosZ proteins (SI Appendix, Fig. S2). (A) The Ca2+ site fixing a loop in the β-propeller domain in mature N2O reductase following Cu insertion. (B) The CuZ site is observed in the [4Cu:2S] state indicative of form I of the enzyme. (C) The dinuclear CuA site in the A chain of the rNosZ dimer. (D) The CuA site in chain B of the dimer. In both cases, ligand His583 is rotated away from ion CuA1, a conformation that likely hinders electron transfer to the metal centers of the enzyme. (E) Stereo representation of the electron density map surrounding the CuA and CuZ sites of rNosZ form I. The blue and green maps are 2Fo − Fc difference electron density maps are contoured at the 1 σ level for the two chains of the homodimer (blue, green), and an anomalous difference Fourier map is shown at the 3.5 σ level (orange), highlighting the lower copper content of the CuZ site with respect to CuA (SI Appendix, Fig. S4).