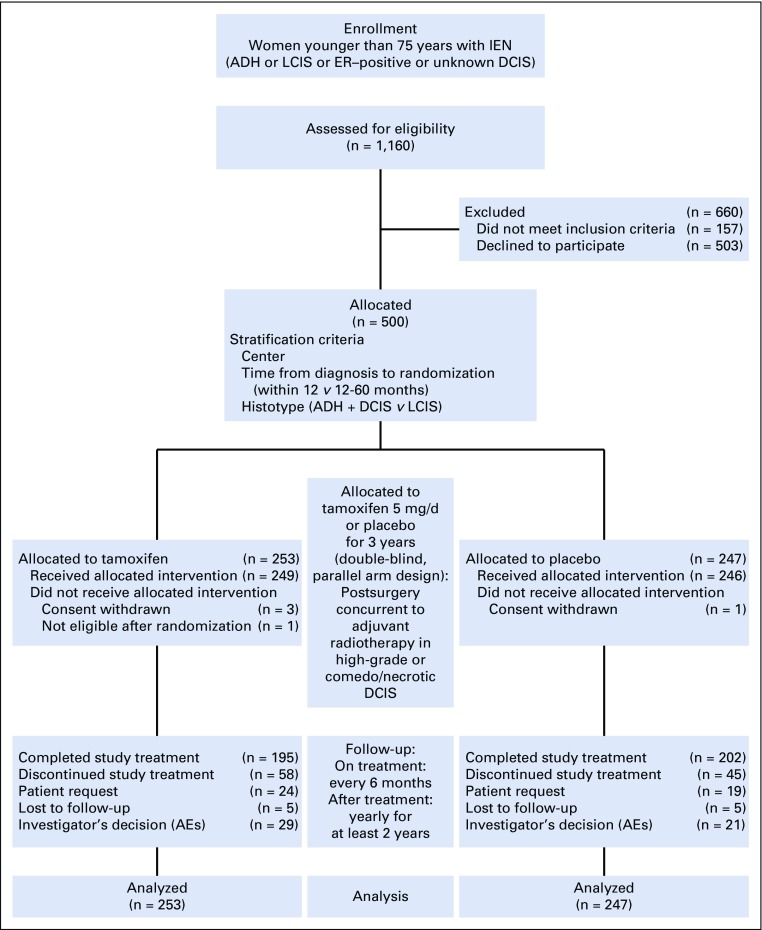
FIG 1.
Participant flow diagram. The intention-to-treat population included all patients who underwent random assignment (n = 253 in the tamoxifen arm; n = 247 in the placebo arm). The safety population included all patients who received at least one dose of trial agent (n = 249 in the tamoxifen arm; n = 246 in the placebo arm). Completion of the study is defined as having completed the 36-month double-blind treatment phase. ADH, atypical ductal hyperplasia; AE, adverse event; DCIS, ductal carcinoma in situ; ER, estrogen receptor; IEN, intraepithelial neoplasia; LCIS, lobular carcinoma in situ.