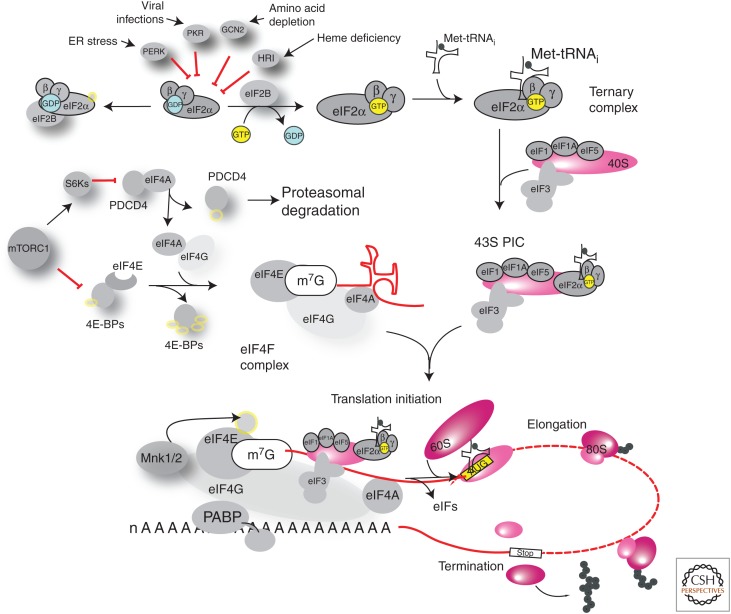
Figure 1.
Overview of translation initiation. Initiation proceeds via a scanning mechanism, whereby the 40S ribosomal subunit is recruited to the 5′ extremity of the messenger RNA (mRNA) and scans the 5′ untranslated region (UTR) of the mRNA toward its 3′ end. When the anticodon of the initiator methionyl-transfer RNA (tRNA) (Met-tRNAi) base-pairs with the start codon, the 60S subunit is recruited and elongation begins with the sequential addition of amino acids until a stop codon is reached and termination occurs. (Top) Formation of the ternary complex (TC): eukaryotic initiation factor (eIF)2—composed of α, β, and γ subunits—GTP and Met-tRNAi. eIF2α can be phosphorylated by protein kinase R (PKR), PERK, GCN2, or HRI, responding to different stresses such as double-stranded RNA, misfolded proteins, amino acid deficiency, and heme deficiency, respectively. Phosphorylation of eIF2α leads to stabilization of the GDP-loaded complex with the guanine nucleotide exchange factor eIF2B and reduced cycling to the active, GTP-bound TC, resulting in inhibition of global protein synthesis and active translation of upstream open reading frame (uORF)-containing mRNAs such as ATF4. TCs associate with the 40S ribosome and other initiation factors, forming the preinitiation complex (PIC). (Middle) The eIF4F complex consists of the cap-binding protein eIF4E, the scaffolding protein eIF4G, and the eIF4A helicase. The eIF4E-binding proteins (4E-BP1/2/3) sequester eIF4E and prevent its binding to eIF4G. Similarly, PDCD4 sequesters eIF4A. Phosphorylation downstream from mammalian target of rapamycin (mTOR) alleviates the inhibitory activity of PDCD4 and 4E-BPs, allowing for eIF4F complex formation, recruitment of the 43S PIC and the initiation of translation. In addition, eIF4E can be phosphorylated by the MNKs.