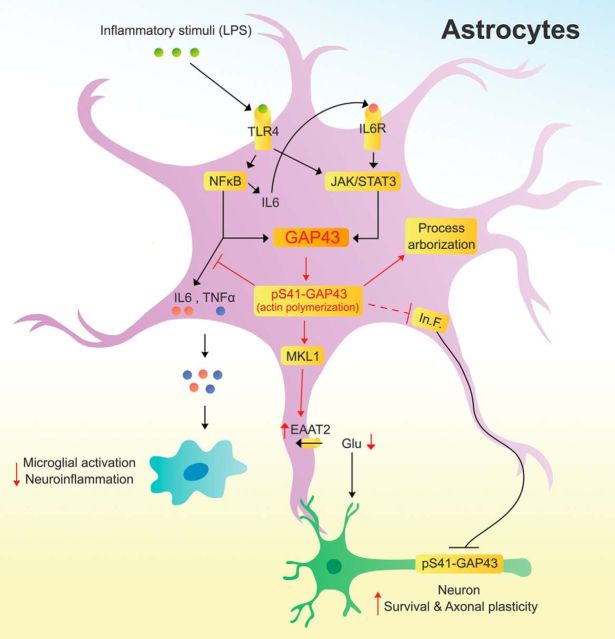
Figure 9.
A proposed model of astrocytic GAP43 functions in mediating astrocytic plasticity; and regulating microglial activation, axonal plasticity, and neuronal survival. In astrocytes, inflammatory stimuli that activate TLR4 trigger both NF-κB and STAT3 signaling pathways and lead to their binding onto the Gap43 gene promoter. NF-κB also mediates the expression and release of IL-6 to activate IL-6 receptor (IL-6R), which then activates a second-phase JAK/STAT3 signaling to give rise to the upregulation of astrocytic GAP43 expression. Astrocytic GAP43, especially its S41-phosphorylated form that promotes actin polymerization, functions to mediate morphological plasticity, including process arborization and elongation in stellate, not flat-shaped, astrocytes during astrogliosis. Furthermore, astrocytic GAP43 may attenuate the release of proinflammatory cytokines (IL-6 and TNF-α), thereby dampening the activation and proinflammatory response in microglia. Astrocytic GAP43 can also facilitate axonal plasticity by attenuating the inhibitory effect of reactivated astrocytes on axon growth and neuronal GAP43 phosphorylation possibly involved the regulation of astrocyte-derived inhibitory factors (In.F.). Finally, an actin-dependent MKL1 activation that contributes to the GAP43-mediated transcriptional activation of EAAT2 was revealed, which increases the glutamate uptake activity and may lead to reduction of excessive glutamate to attenuate the astrogliosis-induced neurotoxicity.