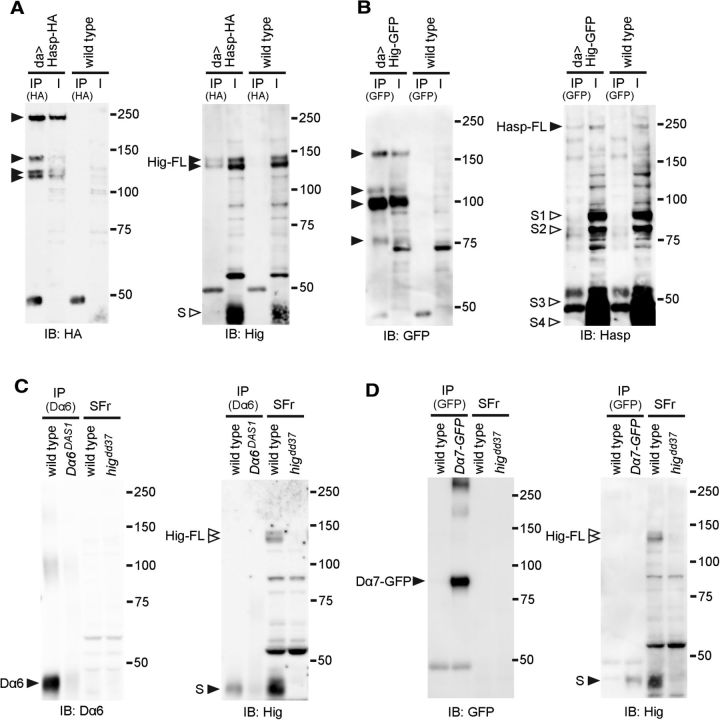
Figure 5.
Hig forms protein complexes with Hasp, Dα6, and Dα7. A, B, Coimmunoprecipitation of Hasp and Hig. Hasp-HA (A) or Hig-GFP (B) was expressed ubiquitously by da-GAL4, and brain extracts were immunoprecipitated with anti-HA beads or anti-GFP beads, respectively. Hasp-HA and endogenous Hig (A), and Hig-GFP and endogenous Hasp (B) in the immunoprecipitated (IP) fractions were detected with the corresponding antibodies. Full-length Hig (two bands) and Hasp were coimmunoprecipitated with Hasp-HA and Hig-GFP, respectively (A,B, right blots). Multiple bands derived from Hasp-HA and Hig-GFP indicate that these tagged proteins were cleaved (A,B, left blots). Extract from wild-type heads was used as a control (right lanes) for each experiment. I, Input; IB, immunoblot. Hasp-HA and Hig-GFP contain the tags at the C terminus, whereas anti-Hasp and anti-Hig antibodies recognize the 108-443 amino acids of Hasp and the 35–295 amino acids of Hig, respectively. Predicted molecular weights of full-length proteins: Hig, 107.0 kDa; Hig-GFP, 133.9 kDa; Hasp, 183.5 kDa; Hasp-HA, 187.3 kDa. The observed molecular weights of these proteins were higher than predicted, suggesting that these proteins are glycosylated. C, Coimmunoprecipitation of Hig and Dα6. Membrane proteins extracted from wild-type or Dα6DAS1 were immunoprecipitated with anti-Dα6 (left blot). The precipitants were further incubated with the soluble fraction extracted from wild-type and examined whether Hig was coimmunoprecipitated with Dα6 (right blot). D, Coimmunoprecipitation of Hig and Dα7-GFP. Membrane proteins extracted from wild-type or flies expressing Dα7-GFP were immunoprecipitated with anti-GFP (left blot). The precipitants were further incubated with the soluble fraction extracted from wild-type, and examined whether Hig was coimmunoprecipitated with Dα7-GFP (right blot). C, D, A short form of Hig (S) was detectable in the IP fraction. SFr represents input of a soluble fraction extracted from wild-type or higdd37. The molecular weight of Dα6 is lower than predicted (59.1 kDa), possibly because of its hydrophobic property. Filled triangles represent the bands of immunoprecipitated polypeptides detected with the antibodies for the tags or proteins shown below each blot. Open triangles represent the absence of the corresponding bands.