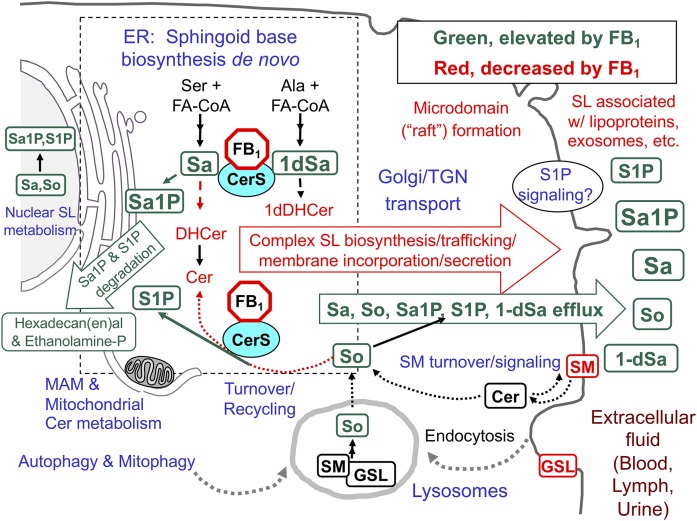
Fig. 2.
An overview of SL biosynthesis and turnover in mammalian cells with highlighted metabolites that are affected by inhibition of CerSs by fumonisins (FB1). The metabolites in green are generally elevated when CerSs are inhibited by FB1 and metabolites in red eventually decrease. The perinuclear dashed box represents the ER, where de novo sphingoid base biosynthesis occurs, with CerSs in the ER, mitochondria, and associated membranes (MAMs). In these reactions, Ser + a fatty acyl-CoA (usually palmitoyl-CoA) are incorporated into 3-ketosphinganine (not shown) and then Sa followed by N-acylation to DHCers by CerS (likewise 1dSa is made from Ala then acylated to 1dDHCer). DHCer is mostly desaturated to Cer and (DH)Cers are incorporated into more complex SLs (mainly SM and GSLs) beginning in the ER lumen (for galactosylCer), cis-Golgi (for glucosylCer, with more complex GSLs made throughout the Golgi apparatus) and trans-Golgi, and plasma membrane (for SM). The SMs and GSLs arrive at their destinations (plasma membrane, exosomes, and other locations) via vesicular transport, transport proteins, and sorting mechanisms at the trans-Golgi network (TGN). SL turnover occurs in multiple locations in the cell (hydrolases in lysosomes, autophagosomes, and other organelles) to release the sphingoid base (mainly So) that is recycled (salvaged) via CerSs or phosphorylated by So kinases to 1-phosphates in multiple locations in the cell. Sphingoid bases (and the 1-phosphates) can function in cell signaling, undergo degradation (to hexadecenal, hexadecanal, and ethanolamine phosphate), or efflux from the cell. Metabolism of Cer from SM in plasma membrane signaling might not be affected by fumonisins until SM is depleted. For more information about these processes, see the references cited in the text and Supplement A.