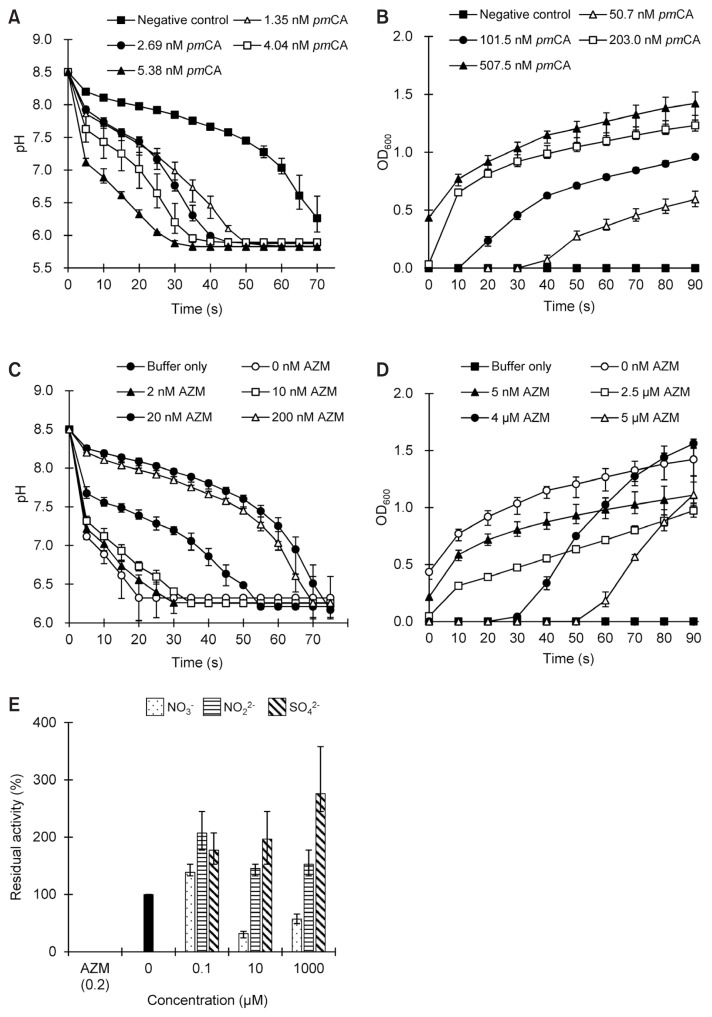
Fig. 1. Enzymatic activity of α-carbonic anhydrase from Persephonella marina EX-H1 (pmCA) and its inhibition by acetazolamide (AZM).
Enzymatic CO2 hydration (A) and CaCO3 precipitation (B), as measured by changes in pH and absorbance at 600 nm (OD600) over time, respectively. For AZM inhibition, protein samples were mixed with varying concentrations of AZM, and changes in pH (C) and OD600 (D) were measured as a function of time, respectively. Reactions without pmCA were prepared as negative controls. (E) Inhibition of pmCA with varying concentrations of anions. The CO2 hydration activity without any inhibitors was used as a positive control, and was set to 100% for comparison. The enzyme activity in the presence of AZM was used as a negative control. Data points indicate the means (± standard deviation [SD]) of triplicate measurements from the same preparation. Error bars indicate SD.