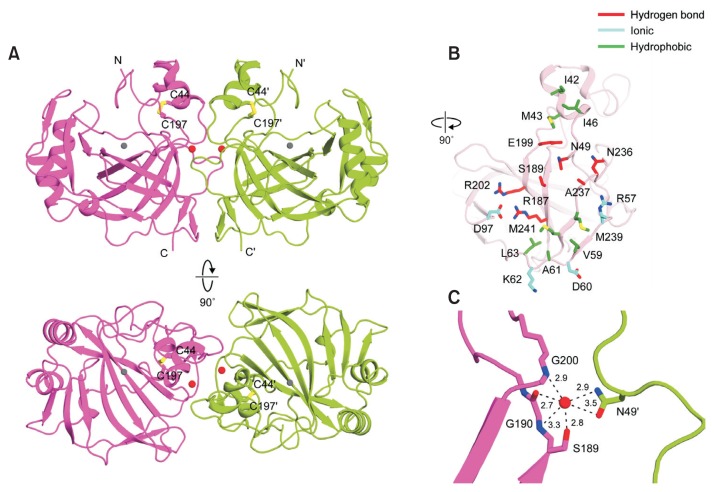
Fig. 2. The crystal structure and dimerization interface of pmCA.
(A) Ribbon representation of the pmCA dimer, comprising two monomers colored magenta and green, respectively. Intramolecular disulfide bonds are shown as yellow sticks, and zinc ions and water molecules are shown as gray and red spheres, respectively. Protein secondary structures were assigned using STRIDE (Heinig and Frishman, 2004). (B) Structure of residues involved in the pmCA dimeric interface. The interface is split and rotated by 90° from Figure 2A, upper panel. The pmCA residues involved in symmetrical hydrogen bonds, ionic, or hydrophobic interactions are colored red, cyan, and green, respectively. (C) The water-mediated hydrogen bond network in the pmCA dimer interface. All distances are shown in angstroms.