Figure 2.
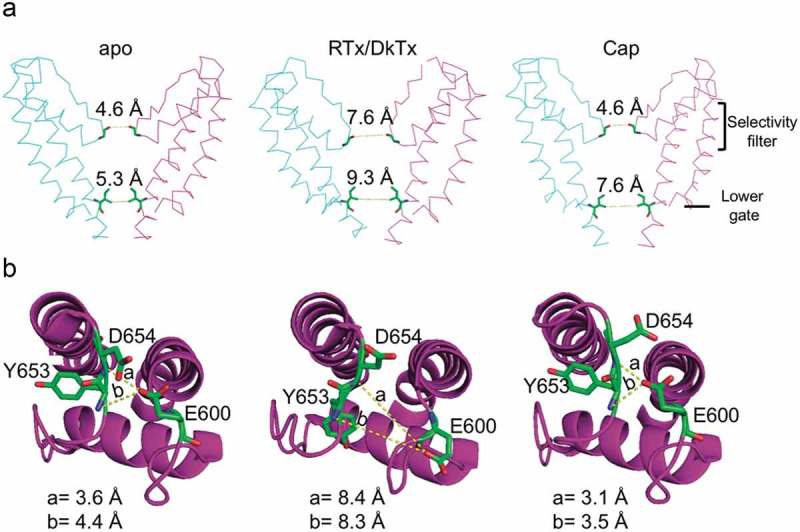
Relative movements of the selectivity filter and lower gate of TRPV1 in the presence of different agonists.
A. Distances between G643 (at the selectivity filter) and I679 (lower gate) in the apo (left, PDB 3J5P), RTx/DkTx (middle, PDB 3J5Q) and capsaicin (right, PDB 3J5R) structures. Compared to the apo structure, in the presence of both RTk and DkTx the selectivity filter and the lower gate expanded. In the structure with capsaicin only (Cap), the outer pore region remains unaltered in comparison with the apo structure, while the lower gate widens. B. Interactions among residues in the outer pore that stabilize the closed conformation of TRPV1. The hydrogen-bonds between E600 side chain with the main-chain nitrogen atoms of Y653 and D654 are broken in the structure with RTk/DkTx but maintained with capsaicin only.
