Abstract
Background:
Alcohol exposure induces TGFβ1 and renders the lung susceptible to injury and disrepair. We determined that TGFβ1 regulates myofibroblast differentiation through the loss of Thy-1 expression and consequent induction of α-SMA. TGFβ1 is important for T helper 17 (Th17) differentiation and IL-17 secretion, which in turn participates in tissue repair. We hypothesized that alcohol induces Th17 differentiation via TGFβ1 and that IL-17 produced by these cells contributes to the development of pro-fibrotic lung myofibroblasts.
Methods:
Primary lung fibroblasts (PLFs) were treated with alcohol, TGFβ1, and IL-17 and then analyzed for Thy-1 expression and cell morphology. Naïve and Th17-polarized CD4+ T cells were exposed to alcohol and assessed for IL-17 expression. CD4+ T cells from alcohol-fed mice were analyzed for Th17 and IL-17 expression. Lungs of control-fed, bleomycin-treated and alcohol-fed, bleomycin-treated mice were analyzed for IL-17 protein expression.
Results:
Alcohol-treated PLFs expressed lower levels of Thy-1 than untreated cells. TGFβ1 or IL-17 exposure suppressed PLF Thy-1 expression. When administered together, TGFβ1 and IL-17 additively downregulated Thy-1 expression. Exposure of naïve and Th17-polarized CD4+ T cells to alcohol induced the Th17 phenotype and augmented their production of IL-17. CD4+ Th17+ levels are elevated in the peripheral compartment but not in the lungs of alcohol-fed animals. Treatment of the PLFs with IL-17 and alcohol induced α-SMA expression. Induction of α-SMA and myofibroblast morphology by IL-17 occurred selectively in a Thy-1− fibroblast subpopulation. Chronic alcohol ingestion augmented lung-specific IL-17 expression following bleomycin-induced lung injury.
Conclusion:
Alcohol exposure skews T cells toward a Th17 immune response that in turn primes the lung for fibroproliferative disrepair through loss of Thy-1 expression and induction of myofibroblast differentiation. These effects suggest that IL-17 and TGFβ1 contribute to fibroproliferative disrepair in the lung and targeting these proteins could limit morbidity and mortality following lung injury in alcoholic individuals.
Keywords: alcohol, myofibroblast, Thy-1, IL-17, TGFβ1
INTRODUCTION
Chronic alcohol ingestion causes significant dysfunction of several organs, including the brain, immune system, liver, and lung (Rehm and Imtiaz, 2016, Kershaw and Guidot, 2008). In the lung, chronic alcohol ingestion independently increases the incidence of acute respiratory distress syndrome (ARDS) ~2–4-fold (Moss et al., 1999). More recently, we showed that chronic alcohol ingestion primes the lung for fibroproliferative disrepair following bleomycin-induced acute lung injury (ALI) in mice (Sueblinvong et al., 2014a). We identified transforming growth factor β1 (TGFβ1) as a proximal and critical mediator of the alcoholic lung phenotype (Sueblinvong et al., 2014c, Bechara et al., 2004, Holguin et al., 1998). Specifically, the lung exhibits increased oxidative stress, TGFβ1 activation, disruption of the epithelial cell barrier, macrophage dysfunction, and fibroproliferative disrepair in response to injury (Bechara et al., 2004, Mehta and Guidot, 2012, Mehta et al., 2011, Sueblinvong et al., 2014a).
Loss of the protein Thy-1 from lung fibroblasts is known to promote fibrotic disrepair by inducing α-SMA and excess collagen deposition (Hagood et al., 2005, Zhou et al., 2004). Recently, we showed that Thy-1 is lost through the epigenetic effects of TGFβ1, a critical factor in tissue repair post-injury (Neveu et al., 2015). In the alcoholic lung, excess baseline TGFβ1 expression is exponentially increased by injury resulting in substantial tissue disrepair (Sueblinvong et al., 2014a, Bechara et al., 2004). Our attempts to restore normal lung tissue homeostasis by blocking epigenetic effects of TGFβ1 in chronic alcohol-exposed lungs improved Thy-1 levels and resulted in partial normalization of fibroblast function (Neveu et al., 2015).
Tissue repair is a complex and tightly regulated process involving multiple cell types, extracellular matrices, and cytokines and results in a replacement of dead or damaged cells to restore normal tissue homeostasis (Gardner et al., 2010). However, when this tightly regulated process is disrupted, unresolved inflammation leads to pathological wound repair and subsequent organ dysfunction (Wynn and Ramalingam, 2012). Importantly, TGFβ1 serves as a bridge between the process of tissue repair and the immune response, as it has been shown to modulate CD4+ T-cell responses to a variety of stimuli (Travis and Sheppard, 2014).
Interleukin-17 (IL-17) has been shown to regulate fibroblast behavior in response to tissue damage, particularly in the bleomycin-induced lung injury rodent model (Park et al., 2018, Cipolla et al., 2017, Wilson et al., 2010). However, the mechanisms by which IL-17 regulates fibroblast behavior remain largely unknown. IL-17 is predominately produced from a subset of effector CD4+ T cells known as T helper 17 (Th17) cells (Korn et al., 2009). Chronic alcohol ingestion increases systemic and local expression of Th17-polarizing cytokines such as IL-6, IL-1β, and IL-23 (Asquith et al., 2014). TGFβ1 has also been shown to promote Th17 activation, and together they promote tissue fibrosis (Hatton, 2011, Fabre et al., 2014). We therefore hypothesized that chronic alcohol ingestion augments the adaptive Th17 immune response and thereby promotes the development of pro-fibrotic lung myofibroblasts, cells which are known to prime the lung for fibroproliferative disrepair following acute lung injury. To test this hypothesis, we evaluated CD4+ T cell responses in a chronic alcohol mouse model and determined the effects of IL-17 on myofibroblast development in the lung.
MATERIALS AND METHODS
Animals and chronic alcohol ingestion
Three-month old C57BL/6J wild type mice were obtained from Jackson Laboratories (Bar Harbor, ME). Utilizing a modified Meadows–Cook alcohol feeding protocol, mice were fed either standard chow with water ad libitum or alcohol as previously described (Sueblinvong et al., 2014a, Spitzer and Meadows, 1999). In brief, mice in the alcohol treatment group were started on 5% ethanol (v/v) in drinking water that was increased by 5% every three days until a final concentration of 20% was achieved. Mice were then continued on 20% ethanol for eight weeks and then treated with intratracheal instillation of bleomycin or euthanized for CD4+ T cell isolation as described below. All studies were approved by the IACUC at Emory University and conformed to institutional standards for the humane treatment of laboratory animals.
Bleomycin-induced acute lung injury
Mice were anesthetized by intraperitoneal injection with Ketamine/Xylazine (100 mg/kg and 10 mg/kg) and bleomycin (2.5 units/kg) (Sigma-Aldrich, St Louis, MO) in phosphate-buffered saline (PBS) or vehicle (PBS) alone was administered intratracheally (Sueblinvong et al., 2014a). At 7 or 14 days after bleomycin injection, the lungs were harvested and processed for Western blot analysis.
Primary lung fibroblast cell culture and treatment
PLFs were isolated from the lungs of 3-month old C57BL/6J (wild-type; Jackson Laboratories) mice as previously described (Sueblinvong et al., 2014a). PLFs (passage three to eight) were seeded in six-well plates at a density of 80,000 cells/ml and cultured in Dulbecco’s Modified Eagle’s Medium (Cellgro, Manassas, VA) supplemented with 20% fetal bovine serum, 100 U/mL penicillin, 100 U/mL streptomycin and 2 mM L-glutamine for 24 hours. Cells were treated with either alcohol (60 mM), TGFβ1 (2 ng/ml; R&D Systems, Minneapolis, MN), or IL-17 (10 ng/ml; R&D Systems). The alcohol dose was selected was based on prior literature demonstrating its relevance in humans (Roman et al., 2005). This dose of alcohol treatment correlates to a blood alcohol level of ~0.24% which is typical of the levels in an inebriated adult. Cell media was changed every 24 hours with freshly added cytokines. At 72 hours, cells were harvested for RNA, protein isolation, and flow cytometry. These time points were selected based on our preliminary experiments and published data in the literature (Neveu et al., 2015, Sueblinvong et al., 2014c).
Lung digestion for single-cell suspensions
Lungs from control-fed and alcohol-fed animals were subjected to enzymatic digestion to obtain single-cell suspensions. Briefly, lung tissues were placed in the petri dish, chopped into small pieces, submerged in 10 ml of digestion solution (collagenase type I (362 units/ml; Sigma-Aldrich, St Louis, MO), DNase (0.05 mg/ml; Sigma-Aldrich, St Louis, MO), and 10% fetal bovine serum in PBS with calcium and magnesium). Samples were incubated at 37°C for 1 hour with frequent agitation. The suspensions were dispersed by aspiration through a blunt needle four times. Cell pellets were resuspended in PBS, filtered with 40 μM cell-strainer and erythrocytes were lysed with ACK lysis buffer (containing ammonium chloride, potassium bicarbonate, and ethylenediaminetetraacetic) at room temperature for 5 minutes. Cells were washed with PBS before isolation of CD4+ T cell isolation as described below.
Thy-1 Subpopulation Purification
Thy-1 positive or negative subpopulations were enriched by immunomagnetic separation using Miltenyi bead system (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were then labeled with anti-PE conjugated with magnetic beads, passed through the magnetic column to be enriched for Thy-1− and Thy-1+ cells per manufacturer’s protocol. Cells were analyzed by flow cytometry as above to confirm the enrichment. Sorted cells were then cultured and expanded in fibroblast culture medium.
CD4+ T cell purification and activation
Lung, splenic, and lymph node (axillary, mesenteric and mediastinal) CD4+ T cells from control-fed and alcohol-fed mice were enriched using biotin-conjugated antibodies from the CD4+ T cell isolation kit according to manufacturer instructions (Miltenyi, San Diego, CA). Isolated CD4+ T cells were seeded in 96-well plates at a density of 5×105 cells/ml and were activated with 5 μg/ml plate-bound anti-CD3 (Clone 145–2C11) and 1 μg/ml soluble anti-CD28 (BD Biosciences, San Jose, CA) monoclonal antibodies for 72 hours. Cell supernatants were collected and stored at −20C until analysis by ELISA. The remaining T cells were washed, counted, and re-stimulated with phorbol 12-myristate 13 acetate (PMA) (5 ng/ml) and Ionomycin (250 ng/ml; Sigma Aldrich) for 6 hours. GolgiStop™ (BD Biosciences) was added to the culture for the final six hours. Cells were harvested for phenotype analysis.
To induce CD4+ Th17 cells, naïve CD4+ T cells from control-fed mice were activated ex vivo with anti-CD3, anti-CD28, recombinant IL-6 (20 ng/ml), and recombinant TGFβ1 (5 ng/ml) for 72 hours (Flaherty and Reynolds, 2015).
Flow cytometry analyses
For CD4+ T phenotype analysis, activated CD4+ T-cells were processed using the CD4+ Th mouse cell phenotyping kit which included IL-17A conjugated with PerCP-Cy™5.5 and CD4+ conjugated with Phycoerythrin (PE) (BD Biosciences).
For Thy-1 expression, Primary lung fibroblasts (PLFs) were stained with an anti-mouse antibody against Thy-1 conjugated with PE (1:50, BioLegend, San Diego, CA). Cells were incubated for 45 min at 4°C, washed in PBS, and fixed in 4% paraformaldehyde for 15 min.
Flow cytometry was performed using a LSRII flow cytometer (Becton Dickinson, San Jose, CA) as previously described (Neveu et al., 2015). Data were analyzed using FlowJo 10.0 software (Tree Star, San Carlos, CA).
Cytokine Detection
Detection of IL-17 in CD4+ T cell culture supernatant was performed using the mouse Duoset ELISA kit according to the manufacturer’s instructions (R&D Systems). Samples were analyzed using the Synergy H1 Multi-mode Reader (Biotek, Winooski, VT).
Quantitative PCR
Activated CD4+ T cells from control-fed and alcohol-fed mice were harvested and processed for RNA extraction at 72 hours using the Zymo Quick-RNA MicroPrep Kit (Zymo, Irvine, CA) as recommended by the manufacturer. First-strand cDNA synthesis was performed as previously described (Sueblinvong et al., 2014a). Quantitative PCR was performed on cDNA using the iCycler iQ Detection System and SYBR Green kit from Bio-Rad Laboratories (Hercules, CA) with primers designed for mouse IL-17A and normalized to β2M levels. Relative values were determined by the comparative Ct method.
Western Blot analyses
Total protein from whole lung lysates and lung fibroblast cell lysates were isolated and protein was analyzed by Western blot as previously described (Sueblinvong et al., 2014a). Blots were probed with rabbit anti-GAPDH (Sigma Aldrich, St. Louis, MO), rabbit anti-α-SMA (Abcam, Cambridge, MA), or rabbit anti-IL17A (Cell Signaling, Danvers, MA) antibodies followed by an appropriate secondary antibody. The immunoreactive bands were visualized using enzyme-linked chemiluminescence and analyzed with the ChemiDoc XRS system (Bio-Rad Laboratories).
Immunofluorescence.
Mouse PLFs were cultured in 4-chamber Nunc Lab-Tek slides ± IL-17 (10 ng/ml) for 96 hours (Thermo Fisher Scientific, Norcross, GA). Cells were fixed, permeabilized, and then stained for α-SMA overnight using rabbit anti-mouse α-SMA antibody at a 1:200 dilution at 4C (Abcam, San Francisco, CA). The chamber slides were incubated with a goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA) and DAPI stain (Vector Labs, Burlingame, CA) and examined using an Olympus BX-41 fluorescence microscope (Olympus, Center Valley, PA). Cell count was performed from four random fields per sample (x20 magnification) from triplicate experiments. Data were expressed as percent of cells with α-SMA stress fiber formation compared with total number of cells.
Statistical analyses.
The data are presented as the mean ± standard error of the mean (SEM). The significance of difference for single and multiple comparisons were determined by the unpaired 2-tailed Student’s t-test and 1-way analysis of variances (ANOVA) followed by Dunnett’s multiple comparisons test, respectively. P-value < 0.05 was considered significant. GraphPad Prism v. 4.03 (La Jolla, CA) was utilized for statistical analysis. All experiments were repeated in triplicate.
RESULTS
Alcohol treatment inhibits Thy-1 expression
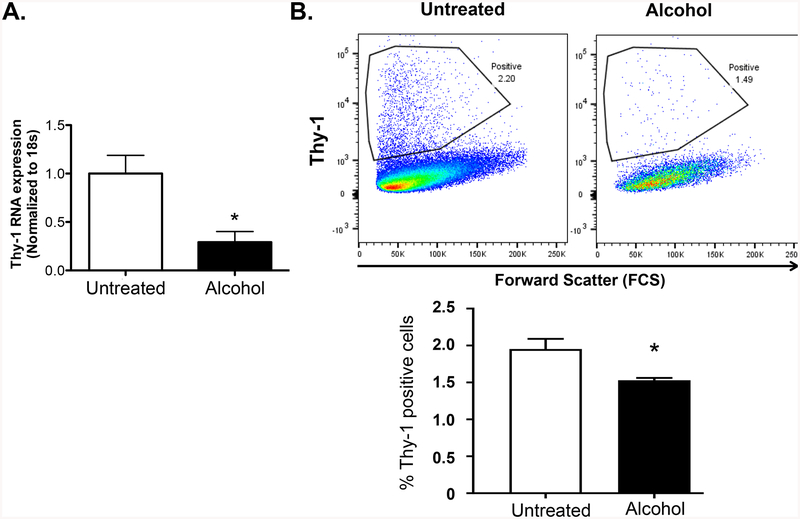
Loss of Thy1 is an earlier step in the process of fibroblast-to-myofibroblast transdifferentiation (Ramirez et al., 2011, Zhou et al., 2004, Hagood et al., 2005). We previously showed that alcohol induces TGFβ1 expression and that TGFβ1 inhibits Thy-1 expression in the lung fibroblasts (Neveu et al., 2015, Sueblinvong et al., 2014c). We speculated that alcohol treatment can inhibit lung fibroblast Thy-1 expression. PLFs were treated with alcohol for 24 or 72 hours before assessment of Thy-1 gene and surface protein expression. Alcohol treatment significantly suppressed Thy-1 gene expression as shown in Figure 1A. In parallel, PLFs cultured with alcohol showed a significant decrease in Thy-1 surface protein expression by flow cytometry (Figure 1B).
Figure 1. Alcohol exposure suppresses Thy-1 expression by lung fibroblasts.
Primary lung fibroblasts (PLFs from passage 3–8) were isolated from wild-type mice and cultured ± alcohol (60 mM) for 24 or 72 hours then analyzed for (A) Thy-1 gene expression by quantitative PCR and (B) Thy-1 surface protein expression by FACS analysis, respectively. *p<0.05 compared to untreated group. N=4–6 per group. Data are presented as mean ± SEM.
IL-17 and TGFβ1 additively inhibit fibroblast Thy-1 expression
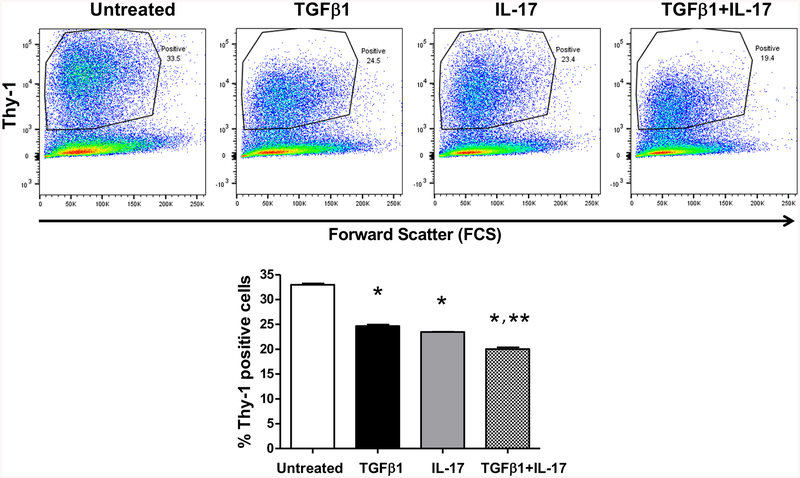
We previously determined that TGFβ1 decreased Thy-1, but the role of immune regulation is less well-characterized. Given the known role of IL-17 in promoting tissue fibrosis, we sought to determine the effects of IL-17 on Thy-1 expression and myofibroblast development. PLFs were cultured with TGFβ1, IL-17, or both for 72 hours prior to analysis of Thy-1 surface expression (Figure 2). As we have previously seen (Neveu et al., 2015), treatment with TGFβ1 significantly downregulates Thy-1 surface expression in PLFs (Figure 2). Similarly, PLFs treated with IL-17 also showed a significant reduction in Thy-1 cell surface expression (Figure 2). Interestingly, the combination of TGFβ1 and IL-17 additively attenuated PLFs Thy-1 expression. (Figure 2). Of note, the differences in the number of Thy-1+ cells in Figure 1 and Figure 2 are likely due to differences in cell passage number, staining efficiency, and flow cytometry settings. These data show that IL-17 and TGFβ1 additively inhibit Thy-1 expression in PLFs and prime fibroblasts for myofibroblast transdifferentiation in vitro.
Figure 2. IL-17 and TGFβ1 independently and additively inhibit lung fibroblast Thy-1 expression.
Primary lung fibroblasts (PLFs from passage 3–8) were isolated from wild-type mice and cultured ± IL-17 (10 ng/ml) ± TGFβ1 (2 ng/ml) for 72 hours prior to analysis for Thy-1 by FACS. *p<0.05 compared to the untreated group, **p<0.05 compared to the TGFβ1-treated group. N=5–6 per group. Data are represented as mean ±SEM.
Alcohol exposure increases Th17 polarization and enhances IL-17 expression by both unpolarized (Th0) and Th17 CD4+ cells in vitro
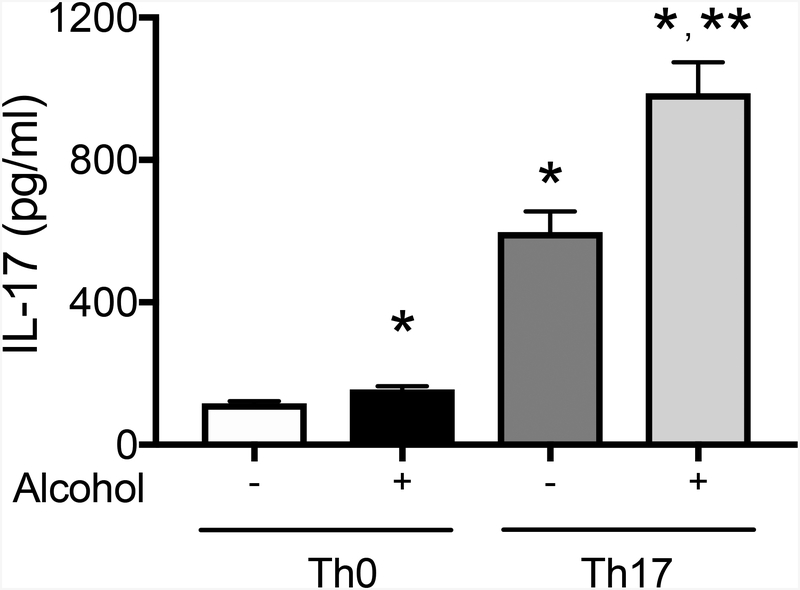
Given that both alcohol and IL-17 expression lead to loss of Thy-1 in fibroblasts, we next sought to determine whether alcohol exposure promotes Th17 development and IL-17 expression. We first activated peripheral CD4+ T cells from naïve C57BL/6J mice with anti-CD3 and anti-CD28 for 72 hours to create Th0 cells. A subgroup of Th0 cells were then polarized to Th17 cells by treating with TGFβ1 at 5 ng/ml and IL-6 at 20 ng/ml for three days. Th0 and Th17 cells were then treated ± alcohol for three days prior to collection of cell supernatant and analysis by ELISA for IL-17 production. Alcohol-treated CD4+ T cells (alcohol-treated Th0) secreted significantly more IL-17 relative to untreated cells (untreated Th0). Additionally, alcohol-treated Th17 cells expressed significantly higher levels of IL-17 when compared to untreated Th0, alcohol-treated Th0, and untreated Th17 cells (Figure 3). These findings suggest that alcohol can directly augment IL-17 production from activated Th17 cells in vitro (Figure 3).
Figure 3. Alcohol exposure induces Th17 differentiation and enhances IL-17 production in both naïve CD4+ T helper cells (Th0) and Th17 cells in vitro.
CD4+ T cells were harvested from the spleen and lymph nodes of three-month-old wild-type mice. Naïve CD4+ T helper cells were activated ex vivo with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) for 72 hours (Th0). A subgroup of Th0 cells were activated and polarized with recombinant IL-6 (20 ng/ml) and recombinant TGFβ1 (5 ng/ml) for 72 hours to generate Th17 cells. Th0 and Th17 CD4+ T cells were cultured ± alcohol (60 mM) for 72 hours. The cell culture supernatant was collected and analyzed for IL-17 production by ELISA. *p<0.05 compared to untreated Th0 and **p<0.05 compared to untreated Th17 cells. N=6 per group. Data are presented as mean ± SEM.
Chronic alcohol exposure increases the systemic, but not lung-specific, Th17 immune response
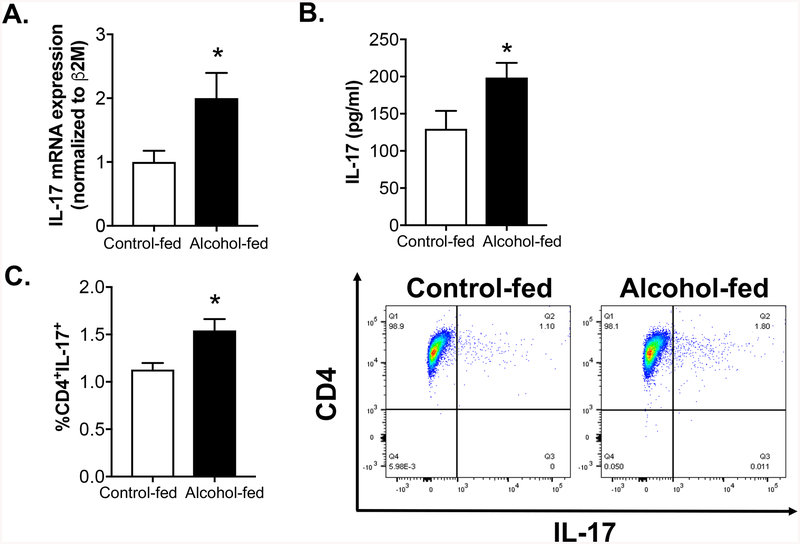
We next evaluated the effect of chronic alcohol ingestion on the systemic Th17 immune response in vivo. Peripheral CD4+ T cells harvested from the spleen and lymph nodes of control-fed and alcohol-fed animals were analyzed for IL-17 expression. CD4+ T cells from alcohol-fed animals expressed significantly higher levels of IL-17 mRNA at baseline compared to CD4+ T cells from control-fed animals (Figure 4A). Consistent with these findings, CD4+ T cells from the alcohol-fed animals produced significantly higher levels of IL-17 than control-fed animals when re-stimulated ex vivo with anti-CD3 and anti-CD28 for 72 hours (Figure 4B). Lastly, the percentages of systemic Th17 cells (CD4+IL-17+) in control-fed and alcohol-fed animals were determined by FACS analysis (Figure 4C). The percentage of Th17 cells from alcohol-fed animals (1.80%) was significantly higher than the percentage of Th17 positive cells in the control-fed animals (1.10%) (Figure 4C).
Figure 4. Chronic alcohol ingestion increases the systemic Th17 immune response.
CD4+ T cells were harvested from the spleen and lymph nodes of three-month-old control-fed and alcohol-fed wild-type mice (20% v/v in drinking water for 8 weeks). (A) Cells were analyzed for IL-17 gene expression by quantitative PCR. CD4+ T cells harvested from control-fed and alcohol-fed animals were activated ex vivo with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) for 72 hours. (B) Cell culture supernatant was analyzed for IL-17 protein expression by ELISA. (C) Lastly, FACS analysis was performed on freshly isolated peripheral CD4+ T cells to determine the percentage of Th17 cells (CD4+IL-17+). The right panel shows representative scattered dot plots. *p<0.05 compared to cells from control-fed animals. N=10 per group. Data are presented as mean ± SEM.
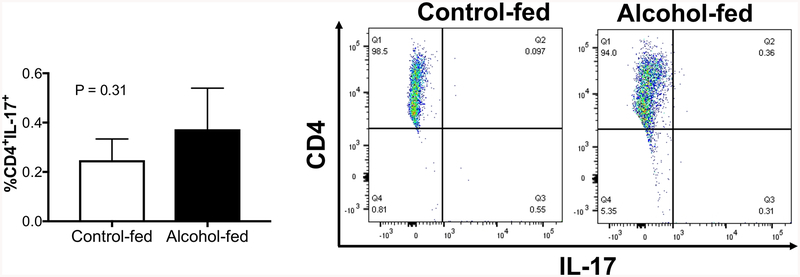
In parallel, we evaluated the effect of chronic alcohol ingestion on the lung specific Th17 immune response in vivo. CD4+ T cells isolated from the lung of uninjured control-fed and alcohol-fed animals were analyzed for IL-17 expression. There was no difference between the percentage of CD4+ IL-17+ cells presence in the lung of uninjured control-fed and alcohol-fed animals (Figure 5). Taken together, these data suggest that, at baseline, chronic alcohol ingestion promotes systemic, but not lung-specific, Th17 development in vivo.
Figure 5. Chronic alcohol ingestion did not alter the CD4+ Th17 population in the lung.
CD4+ T cells were isolated from the lung of three-month-old control-fed and alcohol-fed wild-type mice. FACS analysis was performed on freshly isolated peripheral CD4+ T cells to determine the percentage of Th17 cells (CD4+IL-17+). The right panel shows representative scattered dot plots. N = 3 per group. Data are presented as mean ± SEM.
IL-17 and alcohol upregulate the myofibroblast marker α-SMA in lung fibroblasts
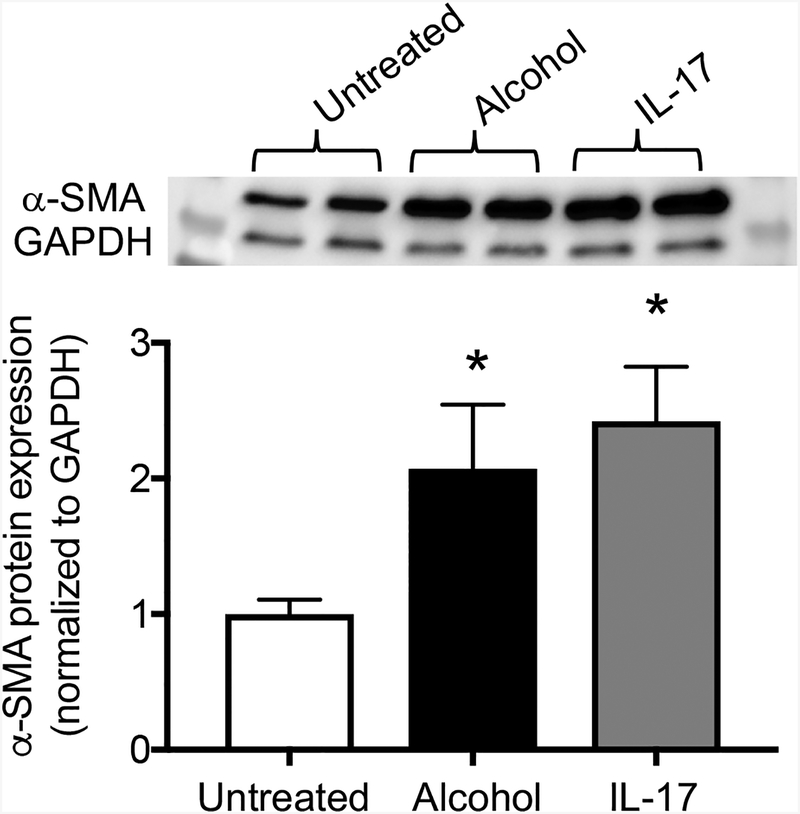
Given that chronic alcohol ingestion induces IL-17 production in T cells and that IL-17 promotes loss of Thy-1 expression in fibroblasts (thereby priming the cells for myofibroblast transdifferentiation), we next sought to determine the effects of alcohol and IL-17 on other myofibroblast markers such as α-SMA. To test this, we treated total lung fibroblasts with either alcohol or IL-17 for 72 hours in vitro. We found that both alcohol and IL-17 significantly upregulated α-SMA protein expression relative to unexposed cells and that there was no statistically significant difference between the treatment groups (Figure 6). These data suggest that both alcohol and IL-17 are sufficient to induce myofibroblast marker expression in lung fibroblasts in vitro.
Figure 6. IL-17 and alcohol upregulate α-SMA in lung fibroblasts.
Total lung fibroblasts (Thy-1+ and Thy-1− subpopulations) were exposed to either alcohol (60 mM) or IL-17 (10 ng/ml) for 72 hours. PLFs were harvested and then analyzed for α-SMA protein expression by Western blot. Untreated cells are represented by the white bar, alcohol-treated cells by the black bar, and IL-17-treated cells by the gray bar. The top panel shows a representative immunoblot. *p<0.05 change compared to untreated group. N=6 per group. Data are presented as mean ±SEM.
The effect of IL-17 on α-SMA expression in lung fibroblasts is mediated through loss of Thy-1 expression.
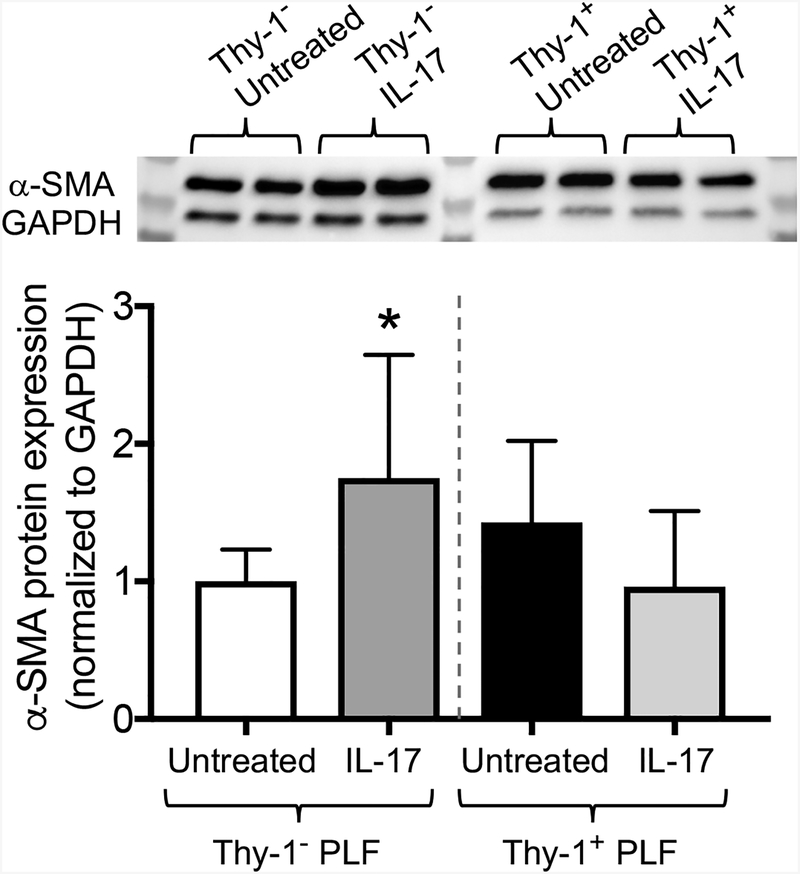
Total lung fibroblasts consist of a mixture of Thy-1 positive (Thy-1+) and Thy-1 negative (Thy-1−) cells. Importantly, Thy-1− PLFs have been shown to be more pro-fibrotic than Thy-1+ PLFs. In addition, we have previously found that the level of Thy-1 expression induces changes in cellular behavior which can affect myofibroblast transdifferentiation. For example, in contrast to the extracellular matrix derived from Thy-1+ PLFs, which induces the fibrocyte cell death signaling pathway, matrix from Thy-1− PLFs induces the fibrocyte survival signaling pathway (Sueblinvong et al., 2014b). We therefore speculated that Thy-1+ and Thy-1− cells would respond differently to IL-17 treatment. To test this, we enriched PLFs for Thy-1+ and Thy-1− subpopulations and separately treated these cells with IL-17 for 72 hours. We found that IL-17 treatment showed a decrease trend in inhibition of α-SMA expression in the Thy-1+ PLFs subset (Figure 7). In contrast, IL-17 treatment significantly induced α-SMA expression in the subset of Thy-1− PLFs (Figure 7). These data suggest that the fibrogenic potential of IL-17 is mediated by Thy-1 cell surface expression.
Figure 7. Thy-1 mediates the effects of IL-17 on α-SMA.
Thy-1 subpopulations were enriched by immunomagnetic separation using the Miltenyi magnetic bead system. Thy-1+ and Thy-1− PLFs were exposed to IL-17 (10 ng/ml) for 72 hours. Thy1− PLFs (white bar (untreated) and dark gray bar (treated)) and Thy-1+ PLFs (black bar (untreated) and light gray bar (treated)) were harvested and then analyzed for α-SMA protein expression by Western blot. The top panel shows representative immunoblots. *p<0.05 change compared to the untreated group. N=6 per group. Data are presented as mean ±SEM.
IL-17 selectively induces myofibroblast development in Thy-1− lung fibroblasts
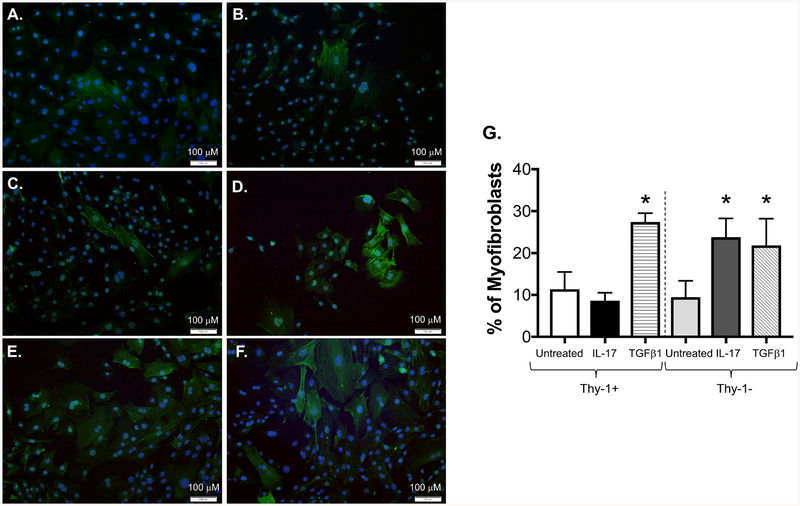
To determine whether IL-17 induces myofibroblast transdifferentiation in Thy-1− PLFs, we separately cultured Thy-1− and Thy-1+ PLFs in the presence or absence of IL-17 and analyzed for cell morphology and α-SMA stress fiber formation by immunofluorescence. At baseline, a similar number of cells express α-SMA in both Thy-1+ and Thy-1− subpopulations (Figure 8A–B). In accordance with our findings above (Figure 7), Thy-1+ PLFs are resistant to IL-17-induced myofibroblast transdifferentiation (Figure 8C) and IL-17 treatment significantly induces myofibroblast transdifferentiation in Thy-1− PLFs (Figure 8D). Figures 8E–F show the results of treatment with TGFβ1 and serves as a positive control, and Figure 8G graphically represents the data collected from four random high-power fields from each cell subset. These data, in conjunction with our Western blot data shown in Figure 7, suggest that Thy-1 expression influences the ability of IL-17 to regulate α-SMA expression and myofibroblast development.
Figure 8. IL-17 selectively induces myofibroblast stress fiber development in Thy-1 negative (Thy-1−) lung fibroblasts.
Thy-1+ and Thy-1− mouse PLFs were separately treated with IL-17 (10 ng/ml) or TGFβ1 (5 ng/ml) for 96 hours. Myofibroblast transdifferentiation was evaluated using immunofluorescent staining for α-SMA (green). DAPI (blue) was used for nuclear staining. Panels (A-B) show untreated Thy-1+ and Thy-1− cells, respectively, (C-D) show Thy-1+ and Thy-1− cells treated with IL-17, respectively, and (E and F) show Thy-1+ and Thy-1− cells treated with TGFβ1, respectively. (G) Four random high-power fields from each sample were analyzed for stress fiber formation. Scale bar = 100 μm. *p < 0.05 compared to untreated Thy-1+ or Thy-1− cells. N = 3 per group. Data are presented as mean ± SEM.
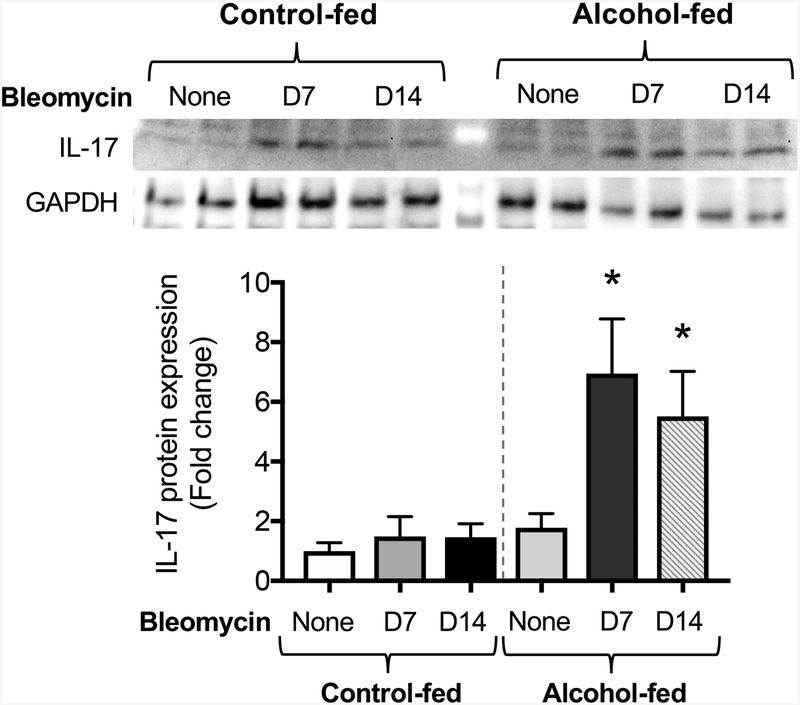
Chronic alcohol ingestion primes the lung for bleomycin augmentation of IL-17
We previously showed that chronic alcohol ingestion primes the lung for fibroproliferative disrepair following bleomycin-induced acute lung injury in an experimental model via upregulation of TGβ1 (Sueblinvong et al., 2014a). It has also been shown that chronic alcohol exposure increases systemic expression of a variety of cytokines (e.g. TGFβ1, IL-6, and IL-23) which are involved in the differentiation and maintenance of Th17 effector T cells (Asquith et al., 2014). Blocking IL-17 protects against fibrosis in the mouse model (Cipolla et al., 2017). We therefore assessed the expression of IL-17 in lungs harvested from control-fed mice and alcohol-fed mice treated with either vehicle or bleomycin. As shown in Figure 9, lungs of control-fed, vehicle-treated mice express a low level of IL-17 and treatment with bleomycin did not upregulate IL-17 expression. In parallel, the lungs of alcohol-fed, vehicle-treated mice increased IL-17 expression (p = 0.06) relative to their control-fed counterparts (Figure 9). However, seven days after bleomycin treatment, the lungs of alcohol-fed, bleomycin-treated mice expressed significantly higher IL-17 levels, corresponding to the acute inflammatory phase. Fourteen days after bleomycin instillation, alcohol-fed mice again demonstrated significantly higher levels of IL-17 than their control-fed counterparts. (Figure 9). Taken together, these data suggest that alcohol exposure augments IL-17 signaling in the injured lung.
Figure 9. Bleomycin-induced acute lung injury augments IL-17 expression in alcohol-fed mice.
Three-month-old wild-type mice were treated ± alcohol (20% v/v in drinking water for 8 weeks) before intratracheal administration of bleomycin (2.5 units/kg) or saline vehicle. Lungs were collected at 7 and 14 days following induction of injury and analyzed for IL-17 protein expression by Western blot. *p<0.05 compared to saline-treated, control-fed mice. The top panel shows representative immunoblots. N=6 per group. Data are presented as mean ±SEM.
DISCUSSION
In this study, we determined that alcohol suppresses Thy-1 levels in murine lung fibroblasts. We also found that TGFβ1 and IL-17 suppress Thy-1 levels, both independently and additively. Alcohol directly induced Th17 development and augmented IL-17 production from activated Th17 cells ex vivo. In parallel, chronic alcohol ingestion promoted Th17 immune development in the systemic compartment but not in the lung-specific compartment. Additionally, lung fibroblasts exposed to alcohol and IL-17 increased α-SMA protein expression and Thy-1 expression mediated the effect of IL-17 on the α-SMA expression by lung fibroblasts. Specifically, Thy-1+ lung fibroblasts are more resistant to IL-17-induced α-SMA expression but Thy-1− lung fibroblasts are susceptible to it. In parallel, IL-17 treatment induced lung fibroblast-to-myofibroblast transdifferentiation in Thy-1− cells but had no effect on Thy-1+ lung fibroblasts. Finally, in a bleomycin model, we found that chronic alcohol ingestion predisposed the murine lung to higher levels of IL-17, priming the lung for scarring in response to acute injury. Taken together, these results provide novel insights into the mechanisms by which chronic alcohol exposure primes the lung for fibroproliferative disrepair following an acute injury.
Following an injury to the lung, resident fibroblasts and epithelial cells are activated, leading to cell proliferation, differentiation, and the release of cytokines and growth factors. In parallel, systemic immune cells traffic to the affected area in response to the injury (Borok et al., 2011). Under normal conditions, these tightly regulated responses lead to the restoration of a functional epithelium without any scar formation. In contrast, the alcoholic lung expresses an excessive amount of TGFβ1 that renders it more susceptible to fibroproliferative disrepair (Sueblinvong et al., 2014a). In that context, this study builds on and complements previous studies by our group and others that have identified that a combination of oxidative stress and activation of TGFβ1 promotes behavioral changes in lung fibroblasts (Neveu et al., 2015, Sueblinvong et al., 2014c, Wynn and Ramalingam, 2012). TGFβ1 has been shown to regulate fibroblast-to-myofibroblast transdifferentiation, which is a critical step in normal wound healing responses and is dysregulated in fibrotic lung diseases (Wynn and Ramalingam, 2012). We previously showed that one of the mechanisms by which TGFβ1 promotes fibroblast-to-myofibroblast transdifferentiation is downregulation of the anti-fibrotic surface protein Thy-1 in lung fibroblasts (Neveu et al., 2015). In addition to its pro-fibrotic effects, TGFβ1 regulates immune responses and promotes Th17 development (Muranski and Restifo, 2013, Hatton, 2011, Travis and Sheppard, 2014). IL-17 is a key cytokine produced by Th17 CD4+ T cells and is an important player in a variety of tissue fibroproliferative disorders affecting the liver, kidney, and lung (Pellicoro et al., 2014, Liu et al., 2015).
Given our prior observations that chronic alcohol ingestion induces and maintains higher TGFβ1 expression in the lung (Bechara et al., 2004, Sueblinvong et al., 2014a, Sueblinvong et al., 2014c) and that TGFβ1 suppresses expression of Thy-1 (Neveu et al., 2015), we first examined the effects of alcohol on Thy-1 expression in lung fibroblasts. We found that alcohol significantly suppressed both Thy-1 gene and surface protein expression in lung fibroblasts. In parallel, given the known relationship between IL-17 and fibrosis, cells were exposed to IL-17 and TGFβ1 and then assessed for Thy-1 expression. IL-17 exposure downregulated lung fibroblast Thy-1 expression but the effects of IL-17 in combination with TGFβ1 were additive in inhibiting Thy-1 expression in lung fibroblasts. These data suggest that the attenuation of Thy-1 expression by IL-17 and TGFβ1 may be an important mechanism by which alcohol primes the lung for disrepair after injury.
Alcohol affects all organs including the lung and the immune system. Previous studies have identified that both acute and chronic binge alcohol exposure alter immune responses (Szabo and Saha, 2015). Because IL-17 is a key protein secreted by CD4+ Th-17 lymphocytes and has been associated with tissue fibrosis, we speculated that chronic alcohol ingestion primes the systemic and/or lung-specific immune system toward pro-fibrotic Th17 responses. To assess that hypothesis, we first assessed the effects of alcohol exposure ex vivo on naïve CD4+ T cells (Th0) and CD4+ Th17 cells and determined that alcohol directly promotes Th17 development and enhances IL-17 production by both naïve CD4+ T cells (Th0) and CD4+ Th17 cells. Next, we examined the CD4+ T cell population from the peripheral compartment and identified more Th17 CD4+ effector T cells in alcohol-fed animals relative to the control group. It has been shown that the changes in low-level circulating T cell populations can be an important means of understanding the adaptive T cell response and immunological memory in various inflammatory diseases. In the absence of an insult, specific T cell frequencies can be less than 1% in both naïve and memory T cell populations (Bacher and Scheffold, 2013). Under the right stimulatory response, existing T cell populations will clonally expand and augment that inflammatory response in the host. Accordingly, a difference in CD4+ Th17 cells of 0.5% in control-fed versus alcohol-fed animals could lead to pathological responses following lung injury. Importantly, we examined the CD4+ T cell population in the lung and found no difference in Th17 CD4+ T cell number in the lung of uninjured control-fed animals versus their alcohol-fed counterparts. Taken together, these findings suggest that chronic alcohol ingestion induces and maintains systemic CD4+ Th17 development at baseline. We speculate that alcohol-induced Th17 development and lung-specific TGFβ1 expression combine to prime the lung for tissue disrepair by altering the phenotype of lung fibroblasts via downregulation of Thy-1. It is possible that following an acute injury, the Th17 CD4+ T cell would be mobilized to the injured tissue (i.e., the lung) leading to an enhancement of Th17 immune response. Because the interaction between Thy-1 and IL-17 is newly described, further research is needed to determine how IL-17 regulates this surface protein and induces a phenotypic/functional change in lung fibroblasts.
As discussed above, fibroblasts lacking Thy-1 expression are involved in the development of lung fibrosis (Hagood et al., 2005, Zhou et al., 2004, Ramirez et al., 2011). Further, Thy-1 expression can dictate how cells respond to stimuli (Sueblinvong et al., 2014b). For instance, extracellular matrix derived from Thy-1− fibroblasts in the presence of TGFβ1 activates cell proliferation and survival pathways (i.e. Akt signaling pathway in fibroblast precursors, fibrocytes) (Sueblinvong et al., 2014b). In contrast, extracellular matrix derived from Thy-1+ fibroblasts in the presence of TGFβ1 activates a programmed cell death pathway (i.e. Caspase signaling pathway) in fibrocytes (Sueblinvong et al., 2014b). We postulated that IL-17 may have differential effects on α-SMA expression and may be dependent on the lung fibroblast Thy-1 expression. By separating the two Thy-1 lung fibroblast subsets, we demonstrated contrasting effects of IL-17 on α-SMA expression and cell morphology. IL-17 induces α-SMA expression and development of the classic myofibroblast “spindle-shaped” morphology in Thy-1− cells whereas IL-17 has no effect on α-SMA expression and myofibroblast development in Thy-1+ cells. The mechanism by which IL-17 differentially regulates α-SMA expression in the presence or absence of Thy-1 is beyond the scope of the current study and requires further investigation.
In the context of our new findings, we further hypothesized that alcohol-induced TGFβ1 expression would promote Th17 type immune responses and prime the lung for fibroproliferative disrepair. To test this hypothesis, we assessed the lung-specific IL-17 expression from chronic alcohol-fed mice with or without bleomycin-induced acute lung injury. Although alcohol ingestion alone was associated with an increase, albeit statistically insignificant, in lung-specific IL-17 expression at baseline, bleomycin-induced acute lung injury caused a significant increase in lung-specific IL-17 that started early in the phase of tissue repair and persisted through the later phase. Consistent with these findings, other groups have found an increase in IL-17 expression in serum from patients with alcoholic liver disease and that the level of IL-17 correlates with the degree of liver fibrosis (Ma et al., 2016). Moreover, chronic alcohol ingestion increased lung specific-Th17 development and IL-17 expression in a post-surgical Klebsiella pneumoniae experimental model (Lanzke et al., 2017). Interestingly, in contrast to a daily chronic alcohol feeding model, there is a downregulation of IL-17 expression 4–6 hours following alcohol ingestion in the lungs of mice in an alcohol binge model (Trevejo-Nunez et al., 2015). These temporal changes in IL-17 expression may be related to a sustained increase in the level of Th17 polarizing cytokines such as IL-6, TGFβ1, and IL-23 that have been identified during chronic alcohol ingestion (Rendon et al., 2012, Bechara et al., 2004) that would not be present after a single alcohol binge.
This study has several limitations. The effects of IL-17 on Thy-1 and α-SMA expression were evaluated in vitro using a cell culture model. Therefore, the potential clinical relevance of these findings will require further investigation in vivo using mouse models of chronic alcohol exposure with either an antibody against IL-17 or in an IL-17 knockout mouse model. Additionally, it will be important to evaluate these pathways in human lung fibroblasts from individuals with chronic alcohol use disorders. Despite wide acceptance that systemic T cell responses reflect tissue specific inflammatory changes in certain diseases (Durham et al., 2000, Zhu et al., 2009), further investigation on the specific effects of alcohol on the Th17 immune response in the lung in response to injury is warranted. Lastly, determining the interplay between TGFβ1 and IL-17 in lung myofibroblast development will be an important step for elucidating signaling pathways that may be of therapeutic potential in fibrotic lung diseases.
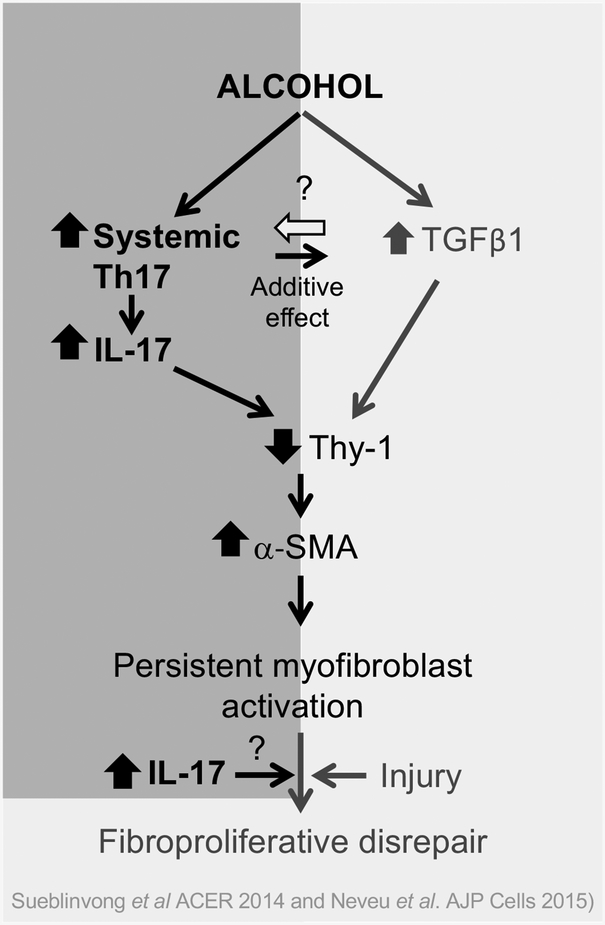
In summary, we determined that chronic alcohol ingestion induces systemic Th17 development and augments IL-17 production. This excessive IL-17, in turn, promotes fibroblast-to-myofibroblast transdifferentiation through inhibition of Thy-1 expression, an effect known to promote fibroproliferative disrepair in the lung. We propose that in individuals with chronic alcohol use disorders that there is an upregulation of Th17 development in the systemic compartment in addition to an elevation of TGFβ1 expression in the lung. This establishes an abnormal environment in which there is aberrant production of IL-17 and TGFβ1 (shown schematically in Figure 10). We speculate that these changes can thereby promote more myofibroblast development leading to tissue disrepair following an injury. The association between IL-17, Thy-1, and myofibroblast development is both novel and provocative. Although it is known that IL-17 is upregulated in fibrotic lung disease, its role was previously unclear. Our studies propose a potential mechanism through which IL-17 may be contributing not only to the alcoholic lung phenotype but to lung disrepair and fibrosis in general. Therefore, future studies investigating how IL-17 regulates myofibroblast development may help elucidate how imbalances in these signaling pathways determine whether the lung experiences healthy repair or a disordered fibrogenic response after an injury.
Figure 10. Hypothesis schematic showing the effects of IL-17 on myofibroblast development in chronic alcohol ingestion.
In the current study, we showed that in the otherwise healthy animals, chronic alcohol ingestion increased systemic Th17 immune response. Acute injury caused a persistent increase in IL-17 in the lung. Alcohol, IL-17, and TGFβ1 independently inhibited Thy-1 expression by lung fibroblasts and IL-17 and TGFβ1 additively decreased Thy-1 expression leading to myofibroblast differentiation. We believe this sequence of events is one of the mechanisms by which alcohol induces fibroproliferative disrepair following acute lung injury.
ACKNOWLEDGMENTS
The authors thank members of David M. Guidot’s lab, including Lucian Marts, MD, Xian Fan, MD, and Abiodun Kukoyi, MD for their helpful scientific discussions and suggestions during the preparation of this manuscript.
This study was supported by NIH K08 AA 021404–01 for VS, NIH R01 AA 017627 for DMG, NIH T32 HL 116271 for WAN, and NIH K08 AA 024512 for BSS.
Footnotes
None of the authors have any conflict of interest to disclose.
REFERENCES:
- Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, Grant KA, Messaoudi I (2014) Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol Clin Exp Res 38:980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher P, Scheffold A (2013) Flow-cytometric analysis of rare antigen-specific T cells. Cytometry A 83:692–701. [DOI] [PubMed] [Google Scholar]
- Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM (2004) Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med 170:188–194. [DOI] [PubMed] [Google Scholar]
- Borok Z, Whitsett JA, Bitterman PB, Thannickal VJ, Kotton DN, Reynolds SD, Krasnow MA, Bianchi DW, Morrisey EE, Hogan BL, Kurie JM, Walker DC, Radisky DC, Nishimura SL, Violette SM, Noble PW, Shapiro SD, Blaisdell CJ, Chapman HA, Kiley J, Gail D, Hoshizaki D (2011) Cell plasticity in lung injury and repair: report from an NHLBI workshop, April 19–20, 2010. Proc Am Thorac Soc 8:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla E, Fisher AJ, Gu H, Mickler EA, Agarwal M, Wilke CA, Kim KK, Moore BB, Vittal R (2017) IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis. FASEB J 31:5543–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham SR, Till SJ, Corrigan CJ (2000) T lymphocytes in asthma: bronchial versus peripheral responses. J Allergy Clin Immunol 106:S221–226. [DOI] [PubMed] [Google Scholar]
- Fabre T, Kared H, Friedman SL, Shoukry NH (2014) IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-beta receptor on hepatic stellate cells in a JNK-dependent manner. J Immunol 193:3925–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty S, Reynolds JM (2015) Mouse Naive CD4+ T Cell Isolation and In vitro Differentiation into T Cell Subsets. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Borthwick LA, Fisher AJ (2010) Lung epithelial wound healing in health and disease. Expert Rev Respir Med 4:647–660. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M (2005) Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol 167:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton RD (2011) TGF-beta in Th17 cell development: the truth is out there. Immunity 34:288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin F, Moss I, Brown LA, Guidot DM (1998) Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 101:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw CD, Guidot DM (2008) Alcoholic lung disease. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism 31:66–75. [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517. [DOI] [PubMed] [Google Scholar]
- Liu T, Dai W, Li C, Liu F, Chen Y, Weng D, Chen J (2015) Baicalin Alleviates Silica-Induced Lung Inflammation and Fibrosis by Inhibiting the Th17 Response in C57BL/6 Mice. J Nat Prod 78:3049–3057. [DOI] [PubMed] [Google Scholar]
- Mehta AJ, Guidot DM (2012) Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci 343:244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Joshi PC, Fan X, Brown LA, Ritzenthaler JD, Roman J, Guidot DM (2011) Zinc supplementation restores PU.1 and Nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcohol Clin Exp Res 35:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M, Steinberg KP, Guidot DM, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE (1999) The effect of chronic alcohol abuse on the incidence of ARDS and the severity of the multiple organ dysfunction syndrome in adults with septic shock: an interim and multivariate analysis. Chest 116:97S–98S. [PubMed] [Google Scholar]
- Muranski P, Restifo NP (2013) Essentials of Th17 cell commitment and plasticity. Blood 121:2402–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu WA, Mills ST, Staitieh BS, Sueblinvong V (2015) TGF-beta1 epigenetically modifies Thy-1 expression in primary lung fibroblasts. Am J Physiol Cell Physiol 309:C616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MJ, Moon SJ, Lee EJ, Jung KA, Kim EK, Kim DS, Lee JH, Kwok SK, Min JK, Park SH, Cho ML (2018) IL-1-IL-17 Signaling Axis Contributes to Fibrosis and Inflammation in Two Different Murine Models of Systemic Sclerosis. Front Immunol 9:1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA (2014) Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 14:181–194. [DOI] [PubMed] [Google Scholar]
- Ramirez G, Hagood JS, Sanders Y, Ramirez R, Becerril C, Segura L, Barrera L, Selman M, Pardo A (2011) Absence of Thy-1 results in TGF-beta induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab Invest 91:1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Imtiaz S (2016) A narrative review of alcohol consumption as a risk factor for global burden of disease. Subst Abuse Treat Prev Policy 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon JL, Janda BA, Bianco ME, Choudhry MA (2012) Ethanol exposure suppresses bone marrow-derived dendritic cell inflammatory responses independent of TLR4 expression. J Interferon Cytokine Res 32:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman J, Ritzenthaler JD, Bechara R, Brown LA, Guidot D (2005) Ethanol stimulates the expression of fibronectin in lung fibroblasts via kinase-dependent signals that activate CREB. Am J Physiol Lung Cell Mol Physiol 288:L975–987. [DOI] [PubMed] [Google Scholar]
- Spitzer JH, Meadows GG (1999) Modulation of perforin, granzyme A, and granzyme B in murine natural killer (NK), IL2 stimulated NK, and lymphokine-activated killer cells by alcohol consumption. Cell Immunol 194:205–212. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Kerchberger VE, Saghafi R, Mills ST, Fan X, Guidot DM (2014a) Chronic alcohol ingestion primes the lung for bleomycin-induced fibrosis in mice. Alcohol Clin Exp Res 38:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueblinvong V, Neveu WA, Neujahr DC, Mills ST, Rojas M, Roman J, Guidot DM (2014b) Aging promotes pro-fibrotic matrix production and increases fibrocyte recruitment during acute lung injury. Adv Biosci Biotechnol 5:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueblinvong V, Tseng V, Smith T, Saghafi R, Mills ST, Neujahr DC, Guidot DM (2014c) TGFbeta1 mediates alcohol-induced Nrf2 suppression in lung fibroblasts. Alcohol Clin Exp Res 38:2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Saha B (2015) Alcohol’s Effect on Host Defense. Alcohol Res 37:159–170. [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Sheppard D (2014) TGF-beta activation and function in immunity. Annu Rev Immunol 32:51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevejo-Nunez G, Chen K, Dufour JP, Bagby GJ, Horne WT, Nelson S, Kolls JK (2015) Ethanol impairs mucosal immunity against Streptococcus pneumoniae infection by disrupting interleukin 17 gene expression. Infect Immun 83:2082–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA (2010) Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207:535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hagood JS, Murphy-Ullrich JE (2004) Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am J Pathol 165:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gadgil AS, Givelber R, George MP, Stoner MW, Sciurba FC, Duncan SR (2009) Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol 182:3270–3277. [DOI] [PubMed] [Google Scholar]