FIGURE 1.
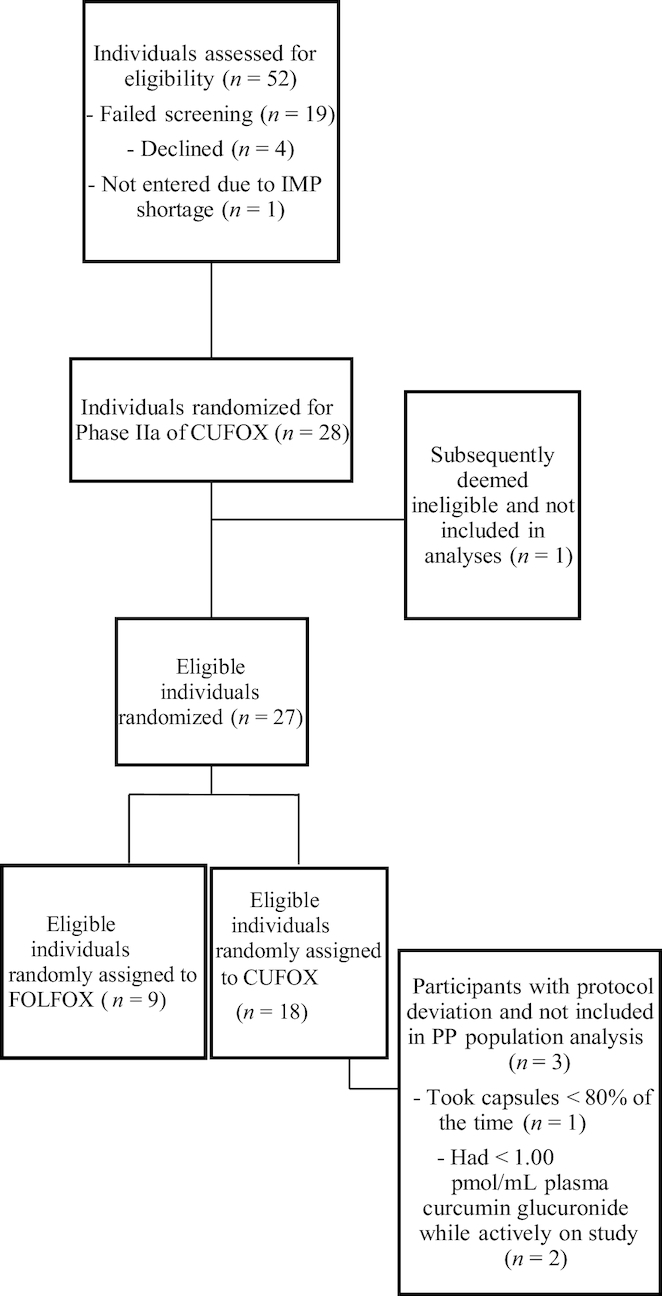
Consolidated Standards of Reporting Trials (CONSORT) diagram showing study participation, random assignment, and PP completion for the CUFOX study. Values represent frequency of events. CUFOX, folinic acid/5-fluorouracil/oxaliplatin + 2 g oral curcumin/d; FOLFOX, folinic acid/5-fluorouracil/oxaliplatin; IMP, investigational medicinal product; PP, per protocol.
