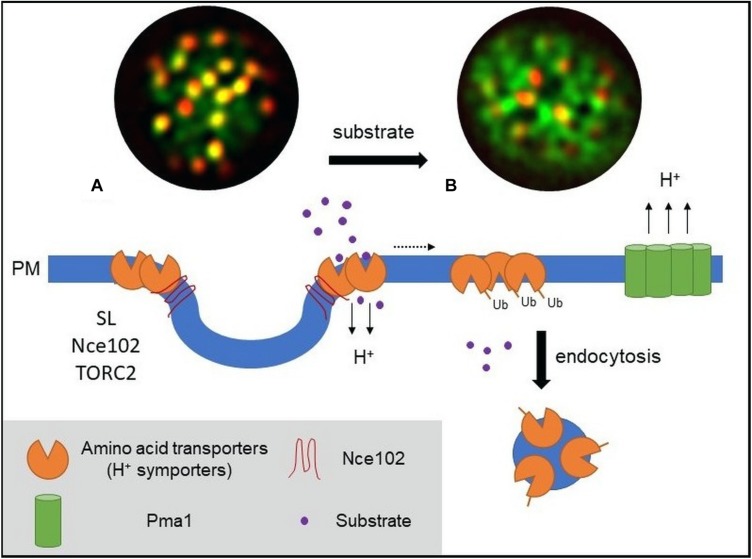
FIGURE 1.
Model of PM amino acid transporter regulation in yeast. (A) Amino acid transporters, generally proton symporters, accumulate at the membrane compartment occupied by Can1 (MCC) during substrate starvation. The MCC represents the edge area of PM furrows (also termed eisosome). Clustering of permeases depends on sphingolipids (SL), the tetraspan protein Nce102, and TORC2 signaling. Clustered transporters adopt an open (outward facing) conformation. (B) Substrate transport triggers a conformational switch to a closed (inward facing) conformation. This switch is linked to lateral relocation of the transporters out of the MCC (dotted arrow) into a unique PM area that is distinct from that occupied by the H+-ATPase Pma1 (MCP). The conformational switch and lateral relocation precede transporter ubiquitination. Ubiquitinated transporters then act as a molecular beacon for the recruitment of the endocytic machinery. Fluorescent images (TIRF) have been adapted from Busto et al. (2018) and represent composites of a ubiquitination-deficient mutant of the methionine permease Mup1 (Mup1-2KR, green) and the eisosomal core component Pil1 (red). Mup1-2KR clusters within the MCC in the absence of methionine (left, colocalization with Pil1 in yellow patches) and relocates out of MCC clusters upon substrate addition.