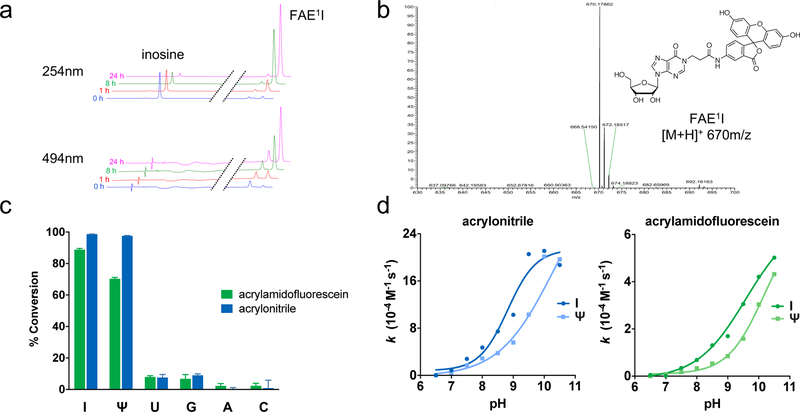
Figure 2.
(a) Representative HPLC traces depicting the reaction between inosine and acrylamidofluorescein over 24 hours. Disappearance of inosine (I) correlates with the appearance of a new putative N1-fluoresceinacrylamidoethylinosine (FAE1I) product peak. (b) ESI-MS analysis confirming mass identity of FAE1I product. (c) Reactivity panel of acrylonitrile and acrylamidofluorescein with ribonucleosides after 24 hours. (d) Dependence of reaction rate constants on pH for the major reacting nucleosides inosine (I) and pseudouridine (Ψ).