Table 1.
Scope of the Ir-catalyzed branched-selective α-alkylation of cyclic ketones with simple alkenes.[a]
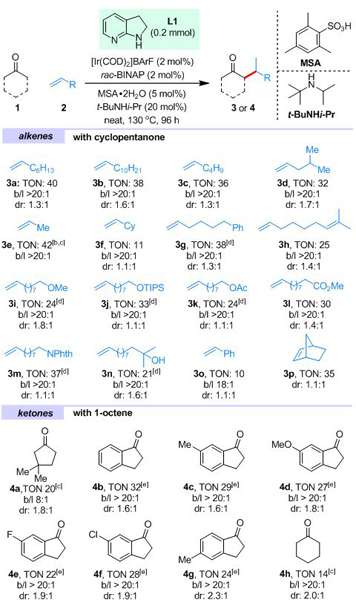 |
Unless otherwise noted, all reactions were run with 0.2 mmol of L1, 0.5 mL of 1 and 0.5 mL of alkene; loadings are based on L1; TONs were calculated based on [Ir] with isolated products; selectivities were determined by 1H NMR or GC-MS of the crude products; b/l = branched:linear ratio.
Pre-condensed propene was used.
The reaction mixture was further treated with 6 M HCl and toluene at 110 °C for 1 h.
2 mmol of alkenes were used.
2 mmol of the ketone substrate was used with 40 mol% of tert-octylamine (instead of t-BuNHi-Pr) based on L1 in 0.3 mL of toluene at 150 °C for 48 h. The reaction was further treated with 6 M HCl at 110 °C for 1 h.
