Abstract
Development of novel disease-modifying treatment strategies for neurological disorders, which at present have no cure, represents a major challenge for today's neurology. Translation of findings from animal models to humans represents an unresolved gap in most of the preclinical studies. Gene therapy is an evolving innovative approach that may prove useful for clinical applications. In animal models of temporal lobe epilepsy (TLE), gene therapy treatments based on viral vectors encoding NPY or galanin have been shown to effectively suppress seizures. However, how this translates to human TLE remains unknown. A unique possibility to validate these animal studies is provided by a surgical therapeutic approach, whereby resected epileptic tissue from temporal lobes of pharmacoresistant patients are available for neurophysiological studies in vitro. To test whether NPY and galanin have antiepileptic actions in human epileptic tissue as well, we applied these neuropeptides directly to human hippocampal slices in vitro. NPY strongly decreased stimulation-induced EPSPs in dentate gyrus and CA1 (up to 30 and 55%, respectively) via Y2 receptors, while galanin had no significant effect. Receptor autoradiographic binding revealed the presence of both NPY and galanin receptors, while functional receptor binding was only detected for NPY, suggesting that galanin receptor signaling may be impaired. These results underline the importance of validating findings from animal studies in human brain tissue, and advocate for NPY as a more appropriate candidate than galanin for future gene therapy trials in pharmacoresistant TLE patients.
Keywords: galanin, gene therapy, hippocampus, NPY, temporal lobe epilepsy
Introduction
One of the major challenges of translational research for brain diseases is how to validate in human specimens the therapeutic outcomes observed in animal models. To this goal, some cases of pharmacoresistant epilepsies, particularly temporal lobe epilepsy (TLE), where brain tissue-generating seizure activity is surgically resected and can be maintained alive as acute brain slices, provides a unique opportunity for in vitro validation of therapeutic compounds. Two such promising compounds are NPY and galanin, endogenous neuropeptides that exert strong seizure-suppressant effects in animal models (Vezzani et al., 1999; Mazarati et al., 2001). These neuropeptides are currently considered putative candidates for gene therapy in epilepsy (Haberman et al., 2003; Richichi et al., 2004). Such novel treatment strategy for epilepsy addresses a strong unmet need as pharmacoresistant patients comprise 30–40% of all epilepsy cases (Duncan et al., 2006).
NPY is a 36 aa peptide found within subpopulations of GABAergic interneurons throughout the human and rodent brain, including hippocampus (de Quidt and Emson, 1986; Köhler et al., 1986; Morris, 1989; Furtinger et al., 2001). Galanin, consisting of 29 aa in rodents and 30 aa in humans, displays a more scattered distribution, but is present within neurons and fibers in several brain regions of rodents and humans, including the hippocampus (Melander et al., 1986; Kordower et al., 1992; Yoshitake et al., 2004).
Several lines of evidence suggest that NPY and galanin are involved in controlling network excitability in the brain. Knock-out animals for NPY and galanin are more prone than wild-type littermates to develop seizures (Erickson et al., 1996; Mazarati et al., 2000) and seizure-induced cell death following kainate treatment (Baraban et al., 1997; Mazarati et al., 2000), while animals overexpressing NPY (Vezzani et al., 2002) or galanin (Mazarati et al., 2000; Kokaia et al., 2001) are more resistant to seizures. Animals injected with adeno-associated viral vector encoding NPY or galanin into the hippocampus exhibit reduced seizure frequency and total time spent in seizures during status epilepticus induced by kainate administration (Lin et al., 2003; Richichi et al., 2004; Noè et al., 2008). These antiepileptic actions of NPY and galanin appear to be related to their ability to reduce presynaptic glutamate release via activation of Y2 (El Bahh et al., 2005) or GalR1 and GalR2 receptors (Zini et al., 1993; Mazarati et al., 2000, 2004), respectively.
For developing novel translational gene therapy strategies based on neuropeptides, it is important to determine whether NPY and galanin exhibit a seizure-suppressing effect in human epileptic tissue, as has been observed in rodents. Here we investigated the action of galanin and NPY on excitatory neurotransmission in human hippocampal slices derived from pharmacoresistant TLE patients. Our data demonstrate that NPY, but not galanin, suppresses excitatory synaptic transmission onto principal neurons in the human epileptic hippocampus, therefore, suggesting that NPY is a more appropriate choice for future gene therapy strategies in pharmacoresistant TLE patients.
Materials and Methods
Subjects and ethical permits.
Only patients undergoing amygdala-hippocampectomy as a treatment for medically intractable TLE were included. Hippocampal tissue was resected en bloc while amygdala tissue was removed by suction (except in two cases). Patients of either sex were diagnosed before surgery based on seizure semiology, extracranial video EEG recording, neuropsychological testing, and structural MRI. In selected cases, intracranial EEG recording and functional imaging were part of the preoperative investigation. Patient details can be found in Table 1. Written informed consent was obtained from every patient before surgery. The study design was approved by the local Ethical Committee in Lund, Sweden (#212/2007) and Copenhagen, Denmark (H-2-2011-104), and performed in accordance with the Declaration of Helsinki.
Table 1.
Patient overview
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration of epilepsy (years) | 20 | 37 | 48 | 8 | 8 | 4 | 4 | 14 | 31 | 6 | 48 | 29 | 21 |
| Resistance to ≥2 anti-epileptic drugs (AEDs) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| AEDs at surgery | GBP, PHE, LEV | CBZ, VPA, LEV | CBZ, VPA, LEV | LTG, LEV | VPA, LTG, LEV, OXZ | LTG, LEV, VPA, CLO | LCM, VPA, CBZ | ECA, LEV | CBZ, CLO | LTG, CLO | CBZ, LEV, CLO, LTG | LEV, PER | LCM, LEV, LTG |
| Gender | M | M | M | M | M | M | M | F | M | F | F | F | F |
| Age at surgery (years) | 43 | 49 | 49 | 21 | 56 | 17 | 57 | 44 | 45 | 53 | 57 | 39 | 26 |
| Age at epilepsy onset (years) | 23 | 12 | <1 | 13 | 48 | 13 | 53 | 19 | 31 | 47 | 9 | 10 | 5 |
| Seizure frequency (n/month) | 4 | 8 | 7 | 12 | 12 | 120 | 2 | 4 | 4 | 3 | 13 | >30 | 20 |
| Hippocampal pathology | HS | HS | HS | HS | HS | HS | HS | AH | IM | HS | HS | HS | HS |
LTG, lamotrigine; LEV, levetiracetam; VPA, valproic acid; CLO, clobazam; PHE, phenytoin; GBP, gabapentin; CBZ, carbamazepine; OXZ, oxcarbazepine; LCM, lacosamide; ECA, eslicarbazepine acetate; PER, perampanel; HS, hippocampal sclerosis (Wyler grades 3–4); IM, insufficient material; AH, abnormal hippocampus, but does not fulfill criteria for hippocampal sclerosis; M, male; F, female.
Human tissue handling and slice preparation.
In the surgery room and immediately after amygdala-hippocampectomy, the resected tissue was cut into coronal sections of approximately 5 mm thickness. These slices were quickly submerged into a transportation beaker containing ice-cold sucrose-based aCSF continuously oxygenated with carbogen (95% O2 and 5% CO2). This aCSF contained the following (in mm): 200 sucrose, 21 NaHCO3, 10 glucose, 3 KCl, 1.25 NaH2PO4, 1.6 CaCl2, 2 MgCl2, and 2 MgSO4, adjusted to 300–310 mOsm, 7.4 pH. Within 20–80 min, slices of 500 μm thickness were cut in sucrose-based aCSF at 4°C using a VT1200 vibratome (Leica Microsystems), and thereafter transferred to an incubation chamber containing the same solution held at 34°C. After 15–20 min, slices were transferred to another chamber containing slightly different aCSF and were allowed to fully recover for an additional 3 h. The latter aCSF solution was also used for slice perfusion during electrophysiological experiments and contained the following (in mm): 129 NaCl, 21 NaHCO3, 10 glucose, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, and 1.6 CaCl2, adjusted to 300–310 mOsm, 7.4 pH, and constantly oxygenated and maintained at 32–34°C.
Electrophysiology.
Individual slices were placed in a submerged recording chamber and infrared differential interference contrast microscopy was used for visual identification of the neurons and approach of the recording pipette. For whole-cell patch-clamp recordings, glass pipettes were back-filled with a solution containing the following (in mm): 122.5 K-gluconate, 12.5 KCl, 10 KOH-HEPES, 0.2 KOH-EGTA, 2 Mg-ATP, 0.3 Na3GTP, and 8 NaCl, pH 7.2–7.4 (mOsm 290–300) and had a tip resistance of 3–5 MΩ. The series resistance during whole-cell recordings was constantly monitored (15.4 ± 1.0 and 16.8 ± 1.1 MΩ; before and after drug application, respectively; n = 20) and if changed >20% over time, the recordings were excluded from the further analysis. Biocytin (5 mg/ml) was always included in the pipette solution for post hoc identification and morphological reconstruction of the recorded cells. Since induction of spontaneous seizure-like events in human slices is difficult when using submerged slice chambers, we focused on evaluating NPY effects on stimulation-evoked excitatory synaptic transmission.
Synaptic transmission was evoked in either dentate gyrus or CA1 by rectangular current pulses (0.1 ms duration) using a stimulation pipette filled with aCSF (0.5–1 MΩ tip resistance) positioned within the medial perforant path (MPP) or stratum radiatum (Schaffer collaterals), respectively. In dentate gyrus, the field recording pipette was placed in the middle portion of the MPP, whereas whole-cell recordings of granule cells were obtained from stratum granulosum. In CA1, the field-recording pipette was positioned in the same subfield as the stimulation pipette. The stimulation current strength was adjusted to generate ∼40–60% of maximal EPSC in whole-cell recordings or fEPSP in field recordings and was kept constant throughout baseline (at least 10 min), drug application (10 min), and washout period (30–60 min). Synthetic human NPY (1 μm; Schafer-N) or synthetic human galanin (0.5 or 1 μm; Schafer-N; 30 aa C terminal), stored in frozen aliquots, was dissolved in aCSF and applied to slices at a speed of 2–2.5 ml/min. The NPY Y2 receptor antagonist BIIE0246 (Tocris Bioscience) was first dissolved in ethanol (25 mm) and subsequently added to the aCSF at a final concentration of 0.6 μm. Siliconized bottles, tubing, and recording chambers were used to minimize adhesion of neuropeptides to the walls. Interpulse intervals for paired-pulse stimulations were set to 50 ms and were applied at a frequency of 0.067 Hz. A single stimulation was applied 50 ms before a train of high-frequency stimulations (40 Hz, 10 pulses), with the intertrain frequency of 0.0167 Hz. At the end of some recordings, NBQX (50 μm; Tocris Bioscience) was applied to slices for blocking AMPA/kainate receptors to confirm the glutamatergic origin of the evoked fEPSPs. All data were acquired at a sampling rate of 10 kHz using Patchmaster Software and HEKA amplifiers (EPC10 or EPC9).
Data analysis and statistics.
Effects of NPY and galanin on evoked synaptic responses during paired and high-frequency stimulations were analyzed off-line using Fitmaster (HEKA Elektronik) and Igor Pro (WaveMetrics) software. Average EPSC and fEPSP amplitudes and average initial slopes of fEPSP were compared between baseline (1–10 min) and the period with estimated peak peptide effect (6–15 min after wash-in start). For the analysis of average initial slope in the BIIE0246 and BIIE0246 + NPY experiments, the last 5 min of drug application was used. Data from experiments where 0.5 μm or 1 μm galanin was applied were pooled together since no statistical differences between the effects (or rather a lack of the effect) of these two concentrations were detectable. The paired-pulse ratios (PPRs) of fEPSPs and EPSCs were determined by dividing the slope/amplitude of the second response by the slope/amplitude of the first response. High-frequency stimulation-induced fEPSPs and EPSCs were normalized to the initial response induced by a single pulse preceding the train stimulation (see above), and the normalized values were compared before and after the drug application. This comparison was performed for the first pulse (first) and consecutive second, fourth, sixth, and eleventh pulses of the evoked responses during the train stimulation. All data are expressed as means ± SEM and analyzed using Student's paired t tests. The level of statistical significance was set at p < 0.05.
Immunohistochemistry.
After electrophysiological experiments, slices were fixed in 4% paraformaldehyde in PB for 12–24 h and then stored in antifreeze solution (ethylene glycol and glycerol in PB) at −20°C until processing. On the day of immunohistochemistry, slices were washed three times in KPBS, and subsequently incubated in 1% Triton X-100-KPBS overnight. The following day, slices were incubated for 3 h in Alexa 488-conjugated streptavidin (Life Technologies; 1:200) and then washed and mounted on glass slides. Slides were finally coverslipped with DABCO and images were obtained with a Leica TCS SP2 confocal microscope. A block of resected human brain tissue not used for electrophysiology was subjected to hematoxylin and microtubule-associated protein 2 (MAP2) staining for clinical diagnostic purposes in compliance to routine procedures at the Division of Pathology, Lund University Hospital.
NPY and galanin receptor binding autoradiography.
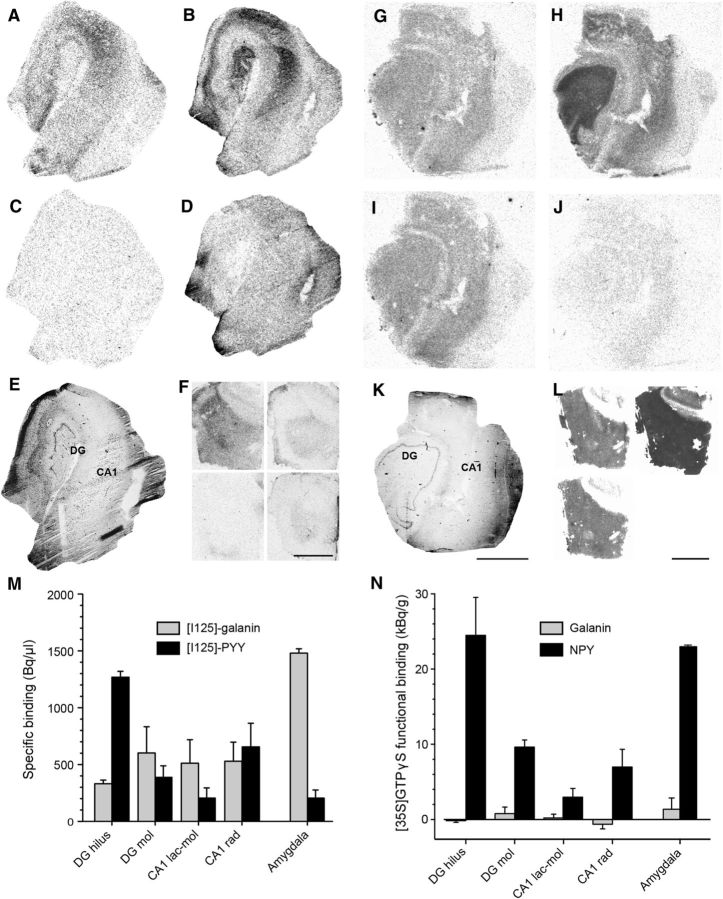
NPY and galanin receptor binding was performed as previously described (Christiansen and Woldbye, 2010; Woldbye et al., 2010). Hippocampal human slices used for electrophysiology were mounted on a cryostat using Cryo-embed (Ax-Lab A/S), cut in serial sections (15 μm thick), thaw mounted onto SuperFrost Plus slides (VWR International), and gently dried on a hotplate. Slides were defrosted at room temperature (RT) and subsequently pre-incubated for 20 min in NPY binding buffer, pH 7.4, containing 25 mm HEPES, 2.5 mm CaCl2, 0.5 g/L bacitracin, 0.5 g/L BSA, or galanin binding buffer, pH 7.4, containing 50 mm Tris-HCl, 5 mm MgCl2, and 2 mm EGTA. Next, slides were incubated at RT for 60 min in NPY binding buffer with the addition of 100 pm [125I]-PYY (#H-6838; Bachem) or galanin binding buffer with the addition of 150 pm porcine synthetic [125I]-galanin (#NEX243010UC; PerkinElmer) along with 0.025% bacitracin, 0.02% leupeptin, and 0.05% BSA. After brief rinsing, NPY-treated slides were washed twice for 30 min in NPY binding buffer at RT and then air dried. Galanin-treated slides were washed twice for 5 min in galanin binding buffer at RT and subsequently air dried. All slides were exposed to [125I]-sensitive Kodak BioMax MS film (Sigma-Aldrich) for 4 d at −20°C together with a standard specimen consisting of different dilutions of the radioligands. Nonspecific binding was estimated by the addition of unlabeled 1 μm synthetic human NPY or human galanin to displace the corresponding radioactive ligand binding. The film was developed in Kodak Processing Chemicals for Autoradiography Films (Sigma-Aldrich). Computer-assisted autoradiographic image analysis was performed using Scion Image (NIH). Measurements were conducted in the hilus and molecular layer of the dentate gyrus, strata lacunosum, and radiatum of CA1 in human slices. Specific [125I]-PYY or [125I]-galanin binding was determined by subtracting nonspecific binding from total binding.
NPY- and galanin-stimulated [35S]-GTPγS functional receptor binding.
Functional binding was performed as previously described (Christensen et al., 2006; Silva et al., 2007). Human brain sections were defrosted for 30 min at RT before being rehydrated for 10 min at RT in assay buffer A (50 mm Tris-HCl, 3 mm MgCl2, 0.2 mm EGTA, and 100 mm NaCl, pH 7.4). Sections were pre-incubated for 20 min at RT in buffer B [assay buffer A, 0.2 mm dithiothreitol, 1 μm 1,3-dipropyl-8-cyclopentylxanthine (#C-101; PerkinElmer), 0.5% w/v BSA, and 2 mm guanosine-5′-diphosphate (#G7127; Sigma-Aldrich)] and then incubated at 25°C for 2 h in buffer B together with 40 pm [35S]-GTPγS (1250 Ci/mmol; #NEG030H250UC; PerkinElmer) and either 1 μm human NPY or galanin (Schafer-N). Basal binding was determined by incubation in buffer B with 40 pm [35S]-GTPγS (1250 Ci/mmol) but without NPY and galanin. Nonspecific binding was determined by incubation in buffer B (without NPY and galanin) with 40 pm [35S]-GTPγS and 10 μm nonlabeled GTPγS (#89378; Sigma-Aldrich). Incubation was terminated by washing twice for 5 min in ice-cold 50 mm Tris-HCl buffer, pH 7.4. Finally, sections were rinsed in ice-cold distilled H2O and dried under a stream of cold air before being exposed to [35S]-sensitive Kodak BioMax MR film together with [14C] microscales for 5 d and then developed in Kodak Processing Chemicals for Autoradiography Films (Sigma-Aldrich). Computer-assisted autoradiographic image analysis and measurements were conducted as described above.
Results
Resected human sclerotic hippocampal slices
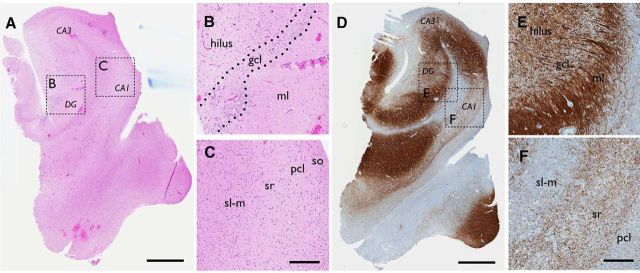
Hippocampal tissue was resected from 13 TLE patients with a medical history of recurrent and pharmacoresistant seizures. Clinical characteristics of patients are summarized in Table 1. Neuropathological examination of the resected tissue by hematoxylin staining revealed severe sclerosis and degeneration within the hippocampal formation in the majority of patients (Fig. 1A–C). In one patient, however, there was insufficient tissue to determine a definite pathology, while another patient did not entirely fulfill the criteria for hippocampal sclerosis (Wyler score of 3–4; Wyler et al., 1992) but had abnormal hippocampus. In tissue with severe sclerosis, a thin granule cell layer was normally observed in the dentate gyrus, and MAP2 immunohistochemical staining suggested substantial mossy fiber sprouting, particularly evident in the supragranular layers (Fig. 1D–F). Significant loss of pyramidal cells was found in CA2-CA3 areas and an almost complete degeneration of pyramidal cells was seen in the CA1 area of the hippocampus (Fig. 1C,F). In the CA1, remaining pyramidal cells did not form a distinct layer but were dispersed. In most cases, due to routine surgery procedures, the resected tissue did not contain the CA3 region of the hippocampus. Therefore, in all slices, field potentials were recorded in the dentate gyrus and CA1, while whole-cell patch-clamp recordings were performed only from dentate granule cells.
Figure 1.
Sprouting and severe degeneration of neurons in hippocampal tissue derived from patients with pharmacoresistant TLE. Example of hematoxylin (A–C) and MAP2 (D–F) staining seen in hippocampal tissue resected from an epileptic patient with severe sclerosis. While the overall architecture of the hippocampus is preserved, the pyramidal cell layer in CA1 is almost completely degenerated with scattered cells seen in surrounding layers (A, C, D, F). The dentate granule cell layer appears intact (A, B), but is accompanied by sprouting (D, E). Boxed areas are magnified. gcl, granule cell layer; ml, molecular layer; so, stratum oriens; pcl, principal cell layer; sr, stratum radiatum; sl-m, stratum lacunosum moleculare. Scale bars: A, D, 1 mm; B, C, E, F, 200 μm.
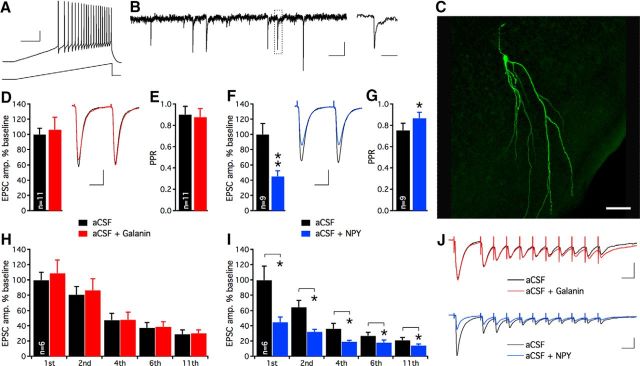
To validate the viability of the resected human tissue, we made one or several whole-cell patch-clamp recordings from dentate granule cells from a subset of the slices. These recordings could be obtained up to 18 h after surgical resection. All recorded dentate granule cells displayed fast action potentials upon current ramp depolarization, and electrophysiological membrane properties (Fig. 2A, Table 2) and postsynaptic currents were highly consistent across slice preparations. Recorded cells displayed functional afferent excitatory synapses revealed by spontaneous EPSCs (Fig. 2B) and EPSCs evoked by electrical stimulation of the perforant path (Fig. 2D,F,J). Biocytin labeling followed by post hoc immunohistochemistry of recorded cells demonstrated morphological features characteristic of dentate granule cells (Fig. 2C). These results show that the resected tissue was viable even a long time after the surgical resection; therefore, it was suitable for electrophysiological experiments.
Figure 2.
Excitatory neurotransmission in dentate granule cells synapses in sclerotic human hippocampal tissue is attenuated by NPY, but not galanin. A, Whole-cell patch-clamp recording of a dentate granule cell in hippocampal slice preparation derived from a TLE patient shows fast repetitive action potentials upon a 300 pA current ramp depolarization. Calibration: 20 mV and 200 ms. B, Spontaneous postsynaptic currents recorded in a dentate granule cell held at −70 mV. Boxed area is magnified on the right. Calibration: 10 pA, 200 and 20 ms, respectively. C, Post hoc visualization of recorded dentate granule cells revealed by immunohistochemistry. Alexa 488-conjugated streptavidin labels intracellular biocytin and shows apical dendrites extending into the molecular layers. Scale bar, 50 μm. D, Galanin application does not affect the amplitude of evoked EPSCs. Insert, Representative traces of evoked EPSCs during aCSF (black trace) and aCSF + galanin application (red trace). Calibration: 50 pA and 20 ms. E, PPR of EPSCs remains unaltered following galanin application. F, NPY application attenuates evoked EPSC amplitudes. Insert, Representative traces of evoked EPSCs during aCSF (black trace) and aCSF + NPY (blue trace) application. Calibration: 50 pA and 20 ms. G, NPY application increases the PPR, suggesting decreased release probability of glutamate. H, I, During 40 Hz stimulation, only NPY suppresses consecutive evoked EPSCs. EPSC amplitudes are normalized to baseline values for each condition. J, Examples of EPSC traces evoked by 40 Hz stimulation shown in aCSF (black), aCSF + galanin (red), and aCSF + NPY (blue), respectively. Calibration: 25 ms, 100 pA; *p < 0.05, **p < 0.01.
Table 2.
Membrane properties of dentate granule cells recorded in slices from TLE patients
| Membrane property | Dentate granule cells (n = 9 cells from 9 patients) |
|---|---|
| Resting membrane potential (mV) | −71.38 ± 0.49 |
| Input resistance (MΩ) | 217.50 ± 9.78 |
| Action potential threshold (mV) | −41.96 ± 0.66 |
| Action potential amplitude (mV) | 92.55 ± 0.58 |
| Action potential duration (ms) | 0.86 ± 0.01 |
Effects of galanin and NPY in human dentate gyrus
In our first series of experiments, we explored if galanin or NPY could suppress evoked synaptic transmission at MPP-dentate granule cell synapses. Whole-cell patch-clamp recordings of dentate granule cells revealed that average EPSC amplitudes and PPR were unaffected by galanin (amplitudes: in aCSF 215 ± 18 pA, in aCSF + galanin: 229 ± 35 pA, n = 11, nonsignificant, paired t test; PPR: in aCSF: 0.90 ± 0.08, in aCSF + galanin: 0.88 ± 0.08, n = 11, n-s, paired t test; Fig. 2D,E), whereas NPY exerted a strong effect (Fig. 2F,G), reducing EPSC amplitudes (in aCSF: 318 ± 46 pA, in aCSF + NPY: 143 ± 24 pA, n = 9, p < 0.01, paired t test) and increasing PPR (in aCSF: 0.75 ± 0.07, in aCSF + NPY: 0.87 ± 0.06, n = 9, p < 0.05, paired t test). These results with NPY are consistent with inhibition of presynaptic glutamate release shown previously in naive, nonepileptic rodents (Colmers et al., 1985, 1988; Qian et al., 1997). At high-frequency stimulations (40 Hz), none of the evoked EPSCs were affected by galanin application (Fig. 2H,J), while NPY strongly suppressed consecutive EPSCs throughout the stimulation train (Fig. 2I,J).
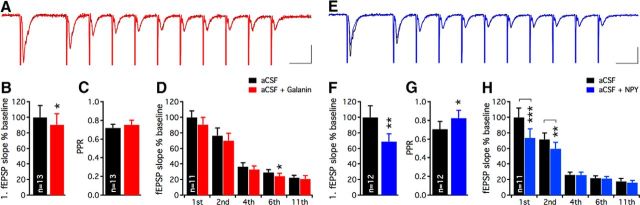
To consolidate these findings, we repeated the same experiment using field recordings (Fig. 3A,E). The slopes of evoked fEPSPs were marginally suppressed by galanin (in aCSF: 100 ± 15.3%, in aCSF + galanin: 90.3 ± 14.3%, n = 13, p < 0.05, paired t test; Fig. 3B), but no change in PPR was observed (in aCSF: 0.75 ± 0.04, in aCSF + galanin: 0.73 ± 0.04, n = 13, nonsignificant, paired t test; Fig. 3C). During 40 Hz stimulations, the effect of galanin was again subtle and only minor suppression of excitatory transmission was detected (Fig. 3D, only sixth pulse). NPY, on the other hand, caused a marked decrease in fEPSPs (in aCSF: 100 ± 15.1%, in aCSF + NPY: 69.2 ± 9.9%, n = 12, p < 0.01, paired t test; Fig. 3F), along with an increase in PPR (in aCSF: 0.71 ± 0.05, in aCSF + NPY: 0.83 ± 0.08, n = 12, p < 0.01, paired t test; Fig. 3G) and a profound reduction in excitatory transmission during the beginning of the 40 Hz stimulation train (Fig. 3H, first and second pulses). Application of NBQX almost completely blocked the fEPSPs (by 95.2 ± 1.2%, n = 9), confirming that MPP stimulation predominantly activated AMPA/kainate receptors in glutamatergic synapses on granule cells.
Figure 3.
Excitatory synaptic transmission onto human dentate granule cells is strongly inhibited by NPY, but not by galanin. Examples of fEPSPs evoked by high-frequency stimulation before and after galanin (A) and NPY (E) application. Merged traces are shown for aCSF (A, black trace) and aCSF + galanin (A, red trace) and aCSF (E, black trace) and aCSF + NPY (E, blue trace) application period, respectively. Calibration: 25 ms, 0.4 mV. Galanin only moderately suppresses the first evoked fEPSP (B), while NPY strongly attenuates the same response (F). fEPSP slopes are normalized to baseline values and compared against the galanin (B) and NPY (F) application period, respectively. PPR of fEPSPs is unaltered by galanin (C), but increased following NPY application (G). During high-frequency stimulation, galanin induces minor effects (significant attenuation at sixth evoked EPSP, D), while NPY prominently suppresses the first two evoked EPSPs (H). Slopes of consecutive fEPSPs are normalized to the first evoked fEPSP during aCSF application. *p < 0.05, **p < 0.01, ***p < 0.001.
Effects of galanin and NPY in human CA1
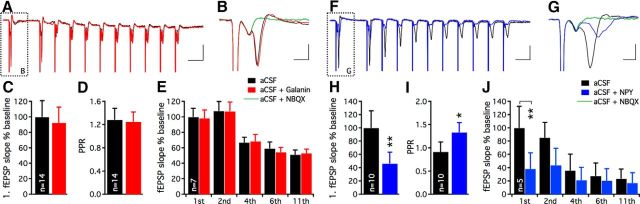
To address whether the limited effect of galanin was specific to dentate gyrus or was more widespread in the hippocampus, similar experiments with field recordings were performed in the CA1 area. Electrical stimulation in stratum radiatum elicited fEPSPs of relatively short duration (Fig. 4A,B,F,G). The relatively short duration of fEPSPs was most likely from degeneration and the dispersion of the remaining CA1 pyramidal cells in the epileptic hippocampus. These fEPSPs were almost completely blocked following NBQX application (96.4 ± 0.8%, n = 12) without affecting the amplitude of the presynaptic fiber volley (Fig. 4B,G), confirming that these postsynaptic potentials were generated by AMPA/kainate receptor activation. Following galanin application, the initial slopes of the fEPSPs observed during single (in aCSF: 100 ± 21.0%, in aCSF + galanin: 92.2 ± 20.5%, n = 14, nonsignificant, paired t test; Fig. 4C) and 40 Hz stimulations (Fig. 4E) remained unchanged. This was also the case for PPR (in aCSF: 1.28 ± 0.20, in aCSF + galanin: 1.25 ± 0.17, n = 14, nonsignificant, paired t test; Fig. 4D). Similar results were found when measuring the amplitude (instead of initial slope) of the fEPSPs (data not shown). In contrast, the initial slopes of fEPSPs were strongly decreased following NPY application (in aCSF: 100 ± 25.8%, in aCSF + NPY: 46.1 ± 17.0%, n = 10, p < 0.01, paired t test; Fig. 4H), and this was accompanied by a significant increase in PPR (in aCSF: 0.91 ± 0.12, in aCSF + NPY: 1.33 ± 0.21, n = 10, p < 0.05, paired t test; Fig. 4I). During 40 Hz stimulations, only the first fEPSP was significantly suppressed, whereas consecutive responses displayed a trend of attenuation following NPY application (Fig. 4J). The inhibitory effect induced by NPY was also confirmed by measuring the amplitudes of the fEPSPs (data not shown). These data suggest that NPY can exert strong inhibitory action on presynaptic glutamate release in the CA1 area of the human epileptic hippocampus, whereas galanin has no effect on glutamatergic transmission.
Figure 4.
Excitatory synaptic transmission in Schaffer collateral-CA1 synapses in human epileptic hippocampus is strongly inhibited by NPY, but not by galanin. Representative traces of high-frequency stimulation-induced fEPSPs in CA1 stratum radiatum during aCSF and following galanin (A) and NPY (F) application. Blocking AMPA/kainate receptors with NBQX almost completely inhibits the postsynaptic potential while the presynaptic fiber volley is intact following galanin (B) and NPY (G) application. Calibrations: A, F, 25 ms, 0.5 mV; B, G, 2 ms, 0.5 mV. C–E, fEPSPs evoked by Schaffer collateral stimulation in CA1 area (C), PPR (D), and consecutive evoked fEPSPs (E) during high-frequency stimulation are unaffected by galanin. During NPY application (H, I), a profound inhibitory effect is observed on the first evoked fEPSP (H, J) with a concomitant increase in PPR (I), indicating suppression of glutamate release. *p < 0.05, **p < 0.01.
Rodent data suggest that it is the Y2 receptor that mediates the inhibitory effects of NPY on hippocampal excitatory transmission (El Bahh et al., 2005). To evaluate whether the Y2 receptor also is important for this effect in human epileptic tissue, we repeated the paired-pulse fEPSP experiments in presence of the highly selective Y2 receptor antagonist BIIE0246 (Doods et al., 1999). Under these conditions, application of NPY to seven hippocampal slices from three TLE patients had no significant effect on fEPSP slopes (in aCSF + BIIE0246: 100 ± 40.1%, in aCSF + BIIE0246 + NPY: 119.2 ± 30.2, paired t test) and PPR (in aCSF + BIIE0246: 1.47 ± 0.25, in aCSF + BIIE0246 + NPY: 1.39 ± 0.33, paired t test) in CA1 (Fig. 5A–C). Two additional field recordings were obtained from dentate gyrus and in both cases the effect of NPY was absent in the presence of BIIE0246 (Fig. 5D). These results clearly suggest that Y2 receptor activation is involved in mediating the inhibitory effect of NPY on presynaptic glutamate release in human CA1 and dentate gyrus.
Figure 5.
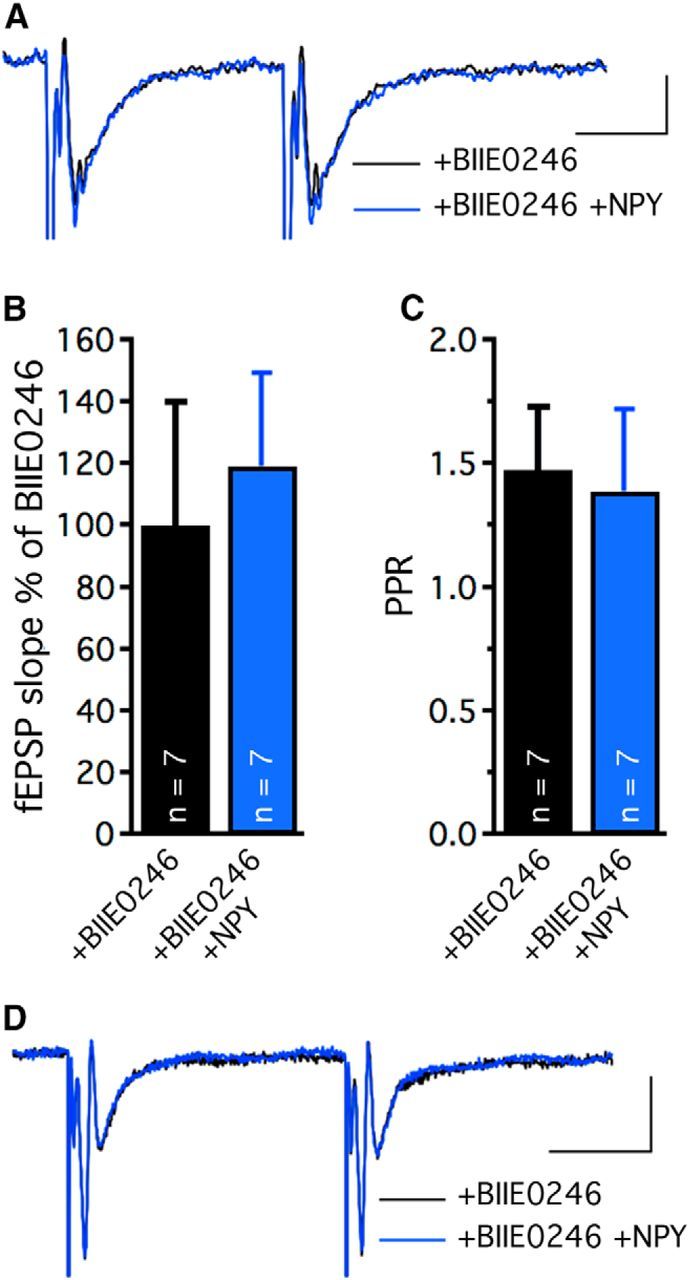
NPY inhibits excitatory transmission in human epileptic hippocampus via Y2 receptors. Representative traces of paired-pulse stimulation-induced fEPSPs in CA1 stratum radiatum (A) or MPP-dentate granule cell synapses (D) before (black) and after (blue) NPY application in presence of the Y2 receptor antagonist BIIE0246. Normalized slopes (B) and PPR (C) of fEPSPs evoked by Schaffer collateral stimulation in CA1 area, showing absence of NPY effect in presence of BIIE0246. Calibrations: A, 20 ms and 0.1 mV; D, 20 ms and 0.4 mV.
Receptor binding and functional binding in human tissue
We speculated that lack of galanin effect in human epileptic tissue could be a consequence of compromised galanin receptor signaling. We found that galanin receptors were expressed within the hilus and molecular layer of the dentate gyrus and in strata lacunosum moleculare and radiatum of CA1, as indicated by specific binding of [125I]-galanin (Fig. 6A,C,M), but the functional receptor binding assay revealed impaired functionality of these receptors (Fig. 6G,I,N). These data suggest that, although galanin receptors are present in the human epileptic hippocampus, their ability for downstream signaling seems to be deficient. In contrast, results from both radioactive ligand binding (Fig. 6B,D,M) and functional binding (Fig. 6H,J,N) assays for NPY indicate that NPY receptors were both present and functional in the human epileptic hippocampus, displaying similar uniform distribution pattern throughout all regions. In human amygdala, radioactive galanin ligand binding levels were substantially higher than in hippocampus and also higher than for NPY (Fig. 6F,M). Such regional differences in galanin receptor binding have also been observed in postmortem human brains with no reported pathologies (Köhler and Chan-Palay, 1990). In these healthy brains, a high density of galanin receptor binding sites was found in the medial and central nucleus of the amygdala, while binding levels in rostral hippocampus were low and undetectable in its caudal aspect (Köhler and Chan-Palay, 1990). Interestingly, despite high levels of galanin receptor binding in TLE tissue, we found that functional receptor binding was still low, suggesting limited signaling ability of these receptors even in amygdala (Fig. 6 L,N), particularly compared with NPY receptor functional binding. To test the sensitivity of our functional galanin binding assay we also used coronal brain sections (15 μm) of the amygdala obtained from naive male Sprague Dawley rats (250 g; n = 4) as a positive control. Indeed, consistent with previous work (Agasse et al., 2013), we were able to clearly demonstrate functional galanin receptor binding in these slices (data not shown), suggesting that the absence of functional galanin binding in human slices was not related to detection problems of our assay. Thus, the data described above indicate that the functionality of galanin receptors may be impaired in the temporal lobe of TLE patients.
Figure 6.
Galanin and NPY receptor binding and functional binding in the human epileptic hippocampus and amygdala. A, Galanin and (B) NPY receptor binding in the human epileptic hippocampus. C, D, Nonspecific binding corresponding to A and B, respectively. E, Hematoxylin staining of an adjacent section showing the gross morphology of the layers analyzed. F, [125I]-galanin binding (top left), corresponding nonspecific binding (bottom left), [125I]-PYY binding (top right), and corresponding nonspecific binding (bottom right) in sections from the human amygdala. G, Galanin and NPY(H) receptor functional binding. I, Basal and nonspecific (J) binding corresponding to G and H, respectively. K, Hematoxylin staining of an adjacent section showing the gross morphology of the layers analyzed. L, Galanin functional binding (top left), NPY functional binding (top right), and corresponding basal binding (bottom left) in sections from the human amygdala. M, Quantification of specific [125I]-galanin and [125I]-PYY receptor binding measured in hippocampal regions (n = 5) and amygdala (n = 2). N, Quantification of galanin and NPY receptor functional binding (i.e., peptide-stimulated binding minus basal binding) measured in hippocampal regions (n = 5) and amygdala (n = 2). Note almost complete absence of galanin receptor functional binding signal (N), despite specific [125I]-galanin binding found in M. Mol, stratum moleculare; rad, stratum radiatum; lac-mol, stratum lacunosum moleculare. Scale bars: A–E, G–K, 3 mm; F, L, 4 mm.
Discussion
Here we demonstrate that resected hippocampus from TLE patients provides a unique possibility to validate, in diseased human brain tissue, the treatment outcomes obtained from animal models. Exogenously applied NPY effectively suppressed excitatory synaptic transmission, while galanin was ineffective. Thus this approach is highly valuable to validate the most effective alternatives for future clinical gene therapy applications. Our data also indicate that downstream signaling of galanin receptors might be impaired in epileptic tissue, which may cause the ineffectiveness of galanin. These findings provide better understanding of the role of neuropeptides in ictogenesis and possibly epileptogenesis, and are important for developing neuropeptide-based gene therapy strategies for epilepsy and potentially other brain disorders associated with hyperexcitability (Rogawski, 2008; Kullmann, 2010; Santos et al., 2013).
The effect of exogenously applied NPY on human epileptic dentate gyrus was explored in one previous publication (Patrylo et al., 1999). In this study, dentate granule cells were recorded with sharp pipettes, while the lateral perforant path (LPP; outer molecular layer) was electrically stimulated. In granule cells, LPP stimulation induced postsynaptic bursting activity, which was effectively attenuated by NPY application. The authors concluded that in human epileptic hippocampus NPY exerts a strong inhibitory effect on presynaptic glutamate release from LPP synapses. Our whole-cell patch-clamp and field recordings demonstrate that glutamate release is inhibited also from MPP synapses by NPY, although we cannot exclude that part of the observed effect may be related to EPSP(C)s generated by sprouted mossy fiber synapses activated by direct stimulation of granule cell dendrites. In addition, we show that glutamate release is also suppressed in Schaffer collateral-CA1 pyramidal cell synapses by NPY, suggesting its widespread inhibitory effect on excitatory transmission in the human epileptic hippocampus. Consistent with these findings, NPY receptors were expressed and functional in both the dentate gyrus and CA1 region as shown by conventional and functional binding assays. Moreover, we show that inhibitory effects of NPY in human hippocampal tissue are mediated by Y2 receptors, which have previously been found to be upregulated in TLE patients (Furtinger et al., 2001).
To our knowledge, possible effects of galanin on synaptic transmission in human brain have not been investigated previously. Our data suggest that galanin has limited action on excitatory synaptic transmission in the human epileptic hippocampus. Studies in animals show that both NPY (Qian et al., 1997; El Bahh et al., 2005) and galanin (Zini et al., 1993; Mazarati et al., 1998, 2000) can act as modulators of excitability by suppressing presynaptic glutamate release, particularly evident in the hippocampus. This is in accordance with several publications demonstrating inhibitory effect of NPY and galanin on seizures in various animal models of epilepsy (for review, see Woldbye and Kokaia, 2004; Lerner et al., 2008; Noè et al., 2009). Attenuation of epileptogenesis in a post status epilepticus model by viral vector-based overexpression of NPY in the hippocampus has also been demonstrated (Noè et al., 2008). Whether neuropeptides can exert similar effect in long-established chronic epileptic seizures is unknown. Our present data from human epileptic hippocampus suggest that only NPY, but not galanin, may be effective in suppressing such chronic seizures.
Why galanin fails to attenuate excitatory synaptic transmission in the human epileptic hippocampus is unclear. Our data show an almost complete absence of functional galanin binding in human epileptic hippocampus, thus indicating deficient galanin signaling. All previously observed seizure-suppressant effects of galanin have been reported in naive, nonepileptic animals, and could therefore still be mediated by unaltered galanin receptor signaling. Alternatively, galanin effects could also be mediated by other mechanisms, partly by suppressing acetylcholine-mediated fEPSPs as shown in CA1 (Fisone et al., 1987; Yoshitake et al., 2011). Apart from presynaptic mechanisms, signaling through postsynaptic G-protein-regulated inwardly rectifying potassium (GIRK) channels that hyperpolarize neurons could also play a role (Smith et al., 1998).
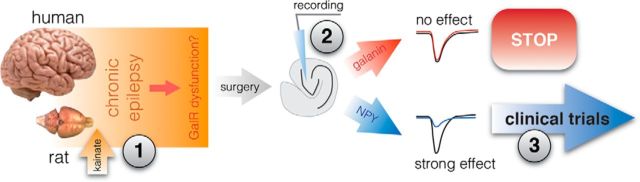
One of the main translational implications of this study is that it suggests a tentative road map for future gene therapy trials in epilepsy. In this regard, our data support the idea that viral vector-based gene therapy approaches to overexpress neuropeptides in the hippocampus should favor NPY rather than galanin as a candidate for therapeutic targeting, particularly if attenuation of glutamatergic excitatory synaptic transmission is an intended objective. In a more general perspective, the results of the present study imply three major points (Fig. 7). (1) Antiepileptic agents need to be tested in epileptic animals. Epileptic conditions may change the target, and the agent that is effective in naive animals may lose its effectiveness in the epileptic brain. (2) The putative candidates need to be tested in human epileptic tissue. Indeed, refractory temporal lobe epilepsy cases provide a unique opportunity to test the novel antiepileptic agents in resected epileptic tissue from patients. This step may reveal the drugs that despite being effective in epileptic animals fail in the human epileptic brain. (3) The “GO/NOGO” decision for clinical trials needs to be based on the outcomes from both epileptic animals and, most importantly, on human epileptic tissue. Clinical trials are rather expensive to perform, and thorough preclinical examination involving human epileptic tissue may decrease the risks for negative outcomes.
Figure 7.
Translational road map for clinical trials with gene therapy in epilepsy. Schematic drawing illustrating the importance of validating results from animal models in human epileptic tissue. Three major points need to be addressed when considering novel therapeutic targets against pharmacoresistant epilepsy. Putative antiepileptic agents need to be tested in epileptic animals with recurrent seizures where their action may differ from that in naive animals (1). The effectiveness of antiepileptic agents needs to be validated in human epileptic tissue, since it may be different from rodent epileptic tissue (2). The decision to continue toward clinical trials needs to be based on results from both animal and human tissue studies to minimize the risk of failure in human trials (3).
Footnotes
This study was supported by grants from Swedish Research Council K2013-61X-14603-11-5, EU Commission FP7 IAPP project EPIXCHANGE, Hardebo's Foundation, and The Danish Council for Independent Research in Medical Sciences (12-127617). A.T.S. and M.L. were supported by The Swedish Brain Foundation Research Fellowships. M.A. was supported by the Swedish Research Council Postdoctoral Fellowship. Assistance and technical support from Laboratory Technician Nora Pernaa is greatly appreciated. We thank Gunnar Skagerberg and Elisabet Englund for valuable contribution on the processing of human tissue.
The authors declare no competing financial interests.
References
- Agasse F, Xapelli S, Coronas V, Christiansen SH, Rosa AI, Sardá-Arroyo L, Santos T, Ferreira R, Schitine C, Harnois T, Bourmeyster N, Bragança J, Bernardino L, Malva JO, Woldbye DP. Galanin promotes neuronal differentiation in murine subventricular zone cell cultures. Stem Cells Dev. 2013;22:1693–1708. doi: 10.1089/scd.2012.0161. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Hollopeter G, Erickson JC, Schwartzkroin PA, Palmiter RD. Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DZ, Olesen MV, Kristiansen H, Mikkelsen JD, Woldbye DP. Unaltered neuropeptide Y (NPY)-stimulated [35S]GTPgammaS binding suggests a net increase in NPY signalling after repeated electroconvulsive seizures in mice. J Neurosci Res. 2006;84:1282–1291. doi: 10.1002/jnr.21028. [DOI] [PubMed] [Google Scholar]
- Christiansen SH, Woldbye DP. Regulation of the galanin system by repeated electroconvulsive seizures in mice. J Neurosci Res. 2010;88:3635–3643. doi: 10.1002/jnr.22517. [DOI] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y reduces orthodromically evoked population spike in rat hippocampal CA1 by a possibly presynaptic mechanism. Brain Res. 1985;346:404–408. doi: 10.1016/0006-8993(85)90880-7. [DOI] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic inhibition. J Neurosci. 1988;8:3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system–II. Immunohistochemical analysis. Neuroscience. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, Engel W, Eberlein W, Rudolf K. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384:R3–R5. doi: 10.1016/S0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- El Bahh B, Balosso S, Hamilton T, Herzog H, Beck-Sickinger AG, Sperk G, Gehlert DR, Vezzani A, Colmers WF. The anti-epileptic actions of neuropeptide Y in the hippocampus are mediated by Y and not Y receptors. Eur J Neurosci. 2005;22:1417–1430. doi: 10.1111/j.1460-9568.2005.04338.x. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Fisone G, Wu CF, Consolo S, Nordström O, Brynne N, Bartfai T, Melander T, Hökfelt T. Galanin inhibits acetylcholine release in the ventral hippocampus of the rat: histochemical, autoradiographic, in vivo, and in vitro studies. Proc Natl Acad Sci U S A. 1987;84:7339–7343. doi: 10.1073/pnas.84.20.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21:5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Samulski RJ, McCown TJ. Attenuation of seizures and neuronal death by adeno-associated virus vector galanin expression and secretion. Nat Med. 2003;9:1076–1080. doi: 10.1038/nm901. [DOI] [PubMed] [Google Scholar]
- Köhler C, Chan-Palay V. Galanin receptors in the post-mortem human brain. Regional distribution of 125I-galanin binding sites using the method of in vitro receptor autoradiography. Neurosci Lett. 1990;120:179–182. doi: 10.1016/0304-3940(90)90032-5. [DOI] [PubMed] [Google Scholar]
- Köhler C, Eriksson L, Davies S, Chan-Palay V. Neuropeptide Y innervation of the hippocampal region in the rat and monkey brain. J Comp Neurol. 1986;244:384–400. doi: 10.1002/cne.902440310. [DOI] [PubMed] [Google Scholar]
- Kokaia M, Holmberg K, Nanobashvili A, Xu ZQ, Kokaia Z, Lendahl U, Hilke S, Theodorsson E, Kahl U, Bartfai T, Lindvall O, Hökfelt T. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proc Natl Acad Sci U S A. 2001;98:14006–14011. doi: 10.1073/pnas.231496298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Le HK, Mufson EJ. Galanin immunoreactivity in the primate central nervous system. J Comp Neurol. 1992;319:479–500. doi: 10.1002/cne.903190403. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Neurological channelopathies. Annu Rev Neurosci. 2010;33:151–172. doi: 10.1146/annurev-neuro-060909-153122. [DOI] [PubMed] [Google Scholar]
- Lerner JT, Sankar R, Mazarati AM. Galanin and epilepsy. Cell Mol Life Sci. 2008;65:1864–1871. doi: 10.1007/s00018-008-8161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EJ, Richichi C, Young D, Baer K, Vezzani A, During MJ. Recombinant AAV-mediated expression of galanin in rat hippocampus suppresses seizure development. Eur J Neurosci. 2003;18:2087–2092. doi: 10.1046/j.1460-9568.2003.02926.x. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Liu H, Soomets U, Sankar R, Shin D, Katsumori H, Langel U, Wasterlain CG. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Langel U, Bartfai T. Galanin: an endogenous anticonvulsant? Neuroscientist. 2001;7:506–517. doi: 10.1177/107385840100700607. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Lu X, Kilk K, Langel U, Wasterlain C, Bartfai T. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur J Neurosci. 2004;19:3235–3244. doi: 10.1111/j.0953-816X.2004.03449.x. [DOI] [PubMed] [Google Scholar]
- Melander T, Staines WA, Rökaeus A. Galanin-like immunoreactivity in hippocampal afferents in the rat, with special reference to cholinergic and noradrenergic inputs. Neuroscience. 1986;19:223–240. doi: 10.1016/0306-4522(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Morris BJ. Neuronal localisation of neuropeptide Y gene expression in rat brain. J Comp Neurol. 1989;290:358–368. doi: 10.1002/cne.902900305. [DOI] [PubMed] [Google Scholar]
- Noè F, Pool AH, Nissinen J, Gobbi M, Bland R, Rizzi M, Balducci C, Ferraguti F, Sperk G, During MJ, Pitkänen A, Vezzani A. Neuropeptide Y gene therapy decreases chronic spontaneous seizures in a rat model of temporal lobe epilepsy. Brain. 2008;131:1506–1515. doi: 10.1093/brain/awn079. [DOI] [PubMed] [Google Scholar]
- Noè F, Frasca A, Balducci C, Carli M, Sperk G, Ferraguti F, Pitkänen A, Bland R, Fitzsimons H, During M, Vezzani A. Neuropeptide Y overexpression using recombinant adeno-associated viral vectors. Neurotherapeutics. 2009;6:300–306. doi: 10.1016/j.nurt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, van den Pol AN, Spencer DD, Williamson A. NPY inhibits glutamatergic excitation in the epileptic human dentate gyrus. J Neurophysiol. 1999;82:478–483. doi: 10.1152/jn.1999.82.1.478. [DOI] [PubMed] [Google Scholar]
- Qian J, Colmers WF, Saggau P. Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry. J Neurosci. 1997;17:8169–8177. doi: 10.1523/JNEUROSCI.17-21-08169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richichi C, Lin EJ, Stefanin D, Colella D, Ravizza T, Grignaschi G, Veglianese P, Sperk G, During MJ, Vezzani A. Anticonvulsant and antiepileptogenic effects mediated by adeno-associated virus vector neuropeptide Y expression in the rat hippocampus. J Neurosci. 2004;24:3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA. Common pathophysiologic mechanisms in migraine and epilepsy. Arch Neurol. 2008;65:709–714. doi: 10.1001/archneur.65.6.709. [DOI] [PubMed] [Google Scholar]
- Santos M, D'Amico D, Spadoni O, Amador-Arjona A, Stork O, Dierssen M. Hippocampal hyperexcitability underlies enhanced fear memories in TgNTRK3, a panic disorder mouse model. J Neurosci. 2013;33:15259–15271. doi: 10.1523/JNEUROSCI.2161-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AP, Lourenço J, Xapelli S, Ferreira R, Kristiansen H, Woldbye DP, Oliveira CR, Malva JO. Protein kinase C activity blocks neuropeptide Y-mediated inhibition of glutamate release and contributes to excitability of the hippocampus in status epilepticus. FASEB J. 2007;21:671–681. doi: 10.1096/fj.06-6163com. [DOI] [PubMed] [Google Scholar]
- Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/S0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Michalkiewicz M, Michalkiewicz T, Moneta D, Ravizza T, Richichi C, Aliprandi M, Mulé F, Pirona L, Gobbi M, Schwarzer C, Sperk G. Seizure susceptibility and epileptogenesis are decreased in transgenic rats overexpressing neuropeptide Y. Neuroscience. 2002;110:237–243. doi: 10.1016/S0306-4522(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Kokaia M. Neuropeptide Y and seizures: effects of exogenously applied ligands. Neuropeptides. 2004;38:253–260. doi: 10.1016/j.npep.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Angehagen M, Gøtzsche CR, Elbrønd-Bek H, Sørensen AT, Christiansen SH, Olesen MV, Nikitidou L, Hansen TV, Kanter-Schlifke I, Kokaia M. Adeno-associated viral vector-induced overexpression of neuropeptide Y Y2 receptors in the hippocampus suppresses seizures. Brain. 2010;133:2778–2788. doi: 10.1093/brain/awq219. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Dohan FC, Schweitzer JB, Berry AD. A grading system for mesial temporal pathology (hippocampal sclerosis) from anterior temporal lobectomy. J Epilepsy. 1992;5:220–225. doi: 10.1016/S0896-6974(05)80120-3. [DOI] [Google Scholar]
- Yoshitake T, Wang FH, Kuteeva E, Holmberg K, Yamaguchi M, Crawley JN, Steiner R, Bartfai T, Ogren SO, Hökfelt T, Kehr J. Enhanced hippocampal noradrenaline and serotonin release in galanin-overexpressing mice after repeated forced swimming test. Proc Natl Acad Sci U S A. 2004;101:354–359. doi: 10.1073/pnas.0307042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Savage S, Elvander-Tottie E, Ogren SO, Kehr J. Galanin differentially regulates acetylcholine release in ventral and dorsal hippocampus: a microdialysis study in awake rat. Neuroscience. 2011;197:172–180. doi: 10.1016/j.neuroscience.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Zini S, Roisin MP, Langel U, Bartfai T, Ben-Ari Y. Galanin reduces release of endogenous excitatory amino acids in the rat hippocampus. Eur J Pharmacol. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]