Figure 8.
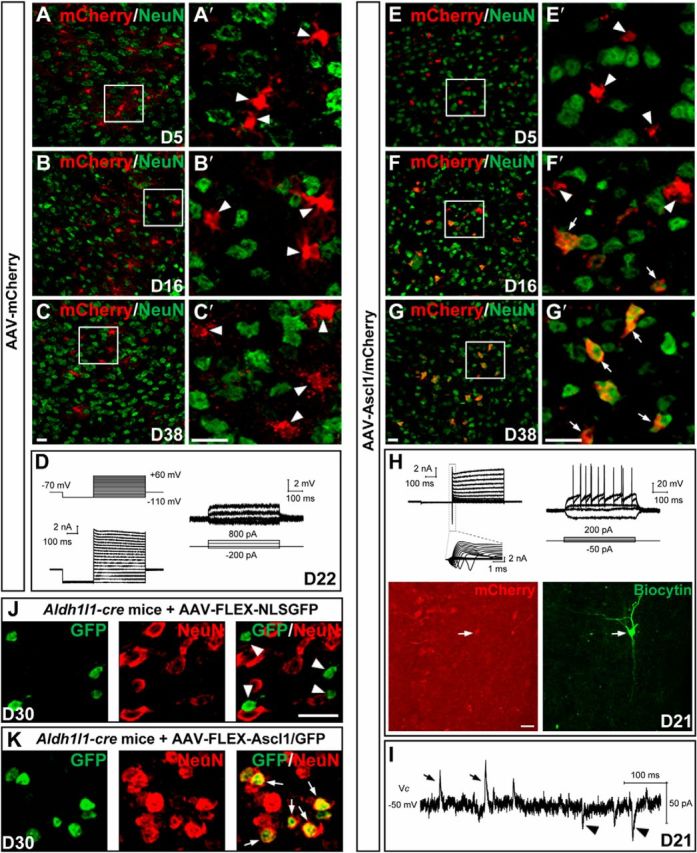
Conversion of dorsal midbrain astrocytes from adult mice into neurons by Ascl1 in vivo. A–C′, Double staining of mCherry and NeuN on sections of the dorsal midbrain from adult mice that were infected with the control virus AAV–mCherry on day 5 (A, A′), day 16 (B, B′), and day 38 (C, C′). mCherry was not colocalized with NeuN (arrowheads). A′, B′, and C′ are higher magnification of the boxed areas in A, B, and C, respectively. D, Membrane functions of a mCherry+ astrocyte (22 DPI) recorded in an acute brain slice of the dorsal midbrain from an adult WT mouse infected with the control virus AAV–mCherry. Membrane currents (left) and voltages (right) were recorded in voltage- and current-clamp modes, respectively, in response to the step voltage or current commands. E–G′, Double staining of mCherry and NeuN on sections of the dorsal midbrain from adult mice that were infected with the virus AAV–Ascl1/mCherry on day 5 (E, E′), day 16 (F, F′), and day 38 (G, G′) after infection. mCherry was gradually colocalized with NeuN (arrows). E′, F′, and G′ are higher magnification of the boxed areas in E, F, and G, respectively. H, Membrane functions of an iN cell (mCherry+) in the slice of dorsal midbrain prepared from an adult WT mouse that was infected with AAV–Ascl1/mCherry at 21 DPI. The arrows in the fluorescence images (mCherry and biocytin staining) indicate the recorded cell. I, Representative trace of spontaneous glutamatergic (inward) and GABAergic (outward) synaptic currents, recorded at Vclamp = −50 mV from an iN cell at 21 DPI. J, Double staining of GFP and NeuN on sections of the dorsal midbrain from adult Aldh1l1–Cre mice on day 30 after infection of the control virus AAV–FLEX–NLSGFP. GFP that was expressed in the nuclei did not colocalize with NeuN (arrowheads). K, Double staining of GFP and NeuN on sections of the dorsal midbrain from adult Aldh1l1–Cre mice on day 30 after infection of the virus AAV–FLEX–Ascl1/GFP. GFP that was expressed in the nuclei colocalized with NeuN (arrows). Scale bars, 20 μm.
