Figure 1.
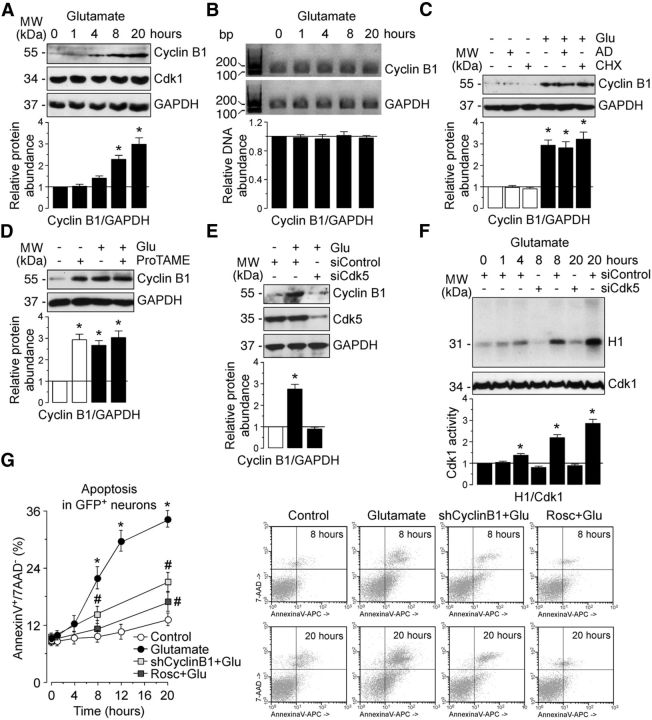
Cyclin B1–Cdk1 activity mediates neuronal apoptotic death on an excitotoxic stimulus. Rat cortical neurons were treated with glutamate (100 μm, 5 min) and were further incubated in culture medium for 1–20 h. A, Western blots of neuronal extracts were probed sequentially with antibodies to cyclin B1 and Cdk1, followed by GAPDH as loading control. B, CyclinB1 and GAPDH mRNA expression was analyzed by 3% agarose electrophoresis after RT-PCR. GAPDH is a housekeeping gene and used as loading control in PCR. One representative gel is shown of three. Shown is the relative cyclin B1 mRNA levels, as normalized with GAPDH, averaged from three independent neuronal cultures (n = 3). After glutamate stimulation (100 μm, 5 min), neurons were incubated in culture medium containing the transcriptional inhibitor ActD (100 ng/ml), the protein synthesis inhibitor CHX (1 μg/ml), or the APC/C inhibitor ProTAME (10 μm) for 20 h. C, Treatment with ActD or CHX did not prevent glutamate-induced cyclin B1 accumulation in neurons. D, ProTAME promoted neuronal cyclin B1 stabilization. E, Neurons on day 4 in vitro were transfected with an siRNA against luciferase (siControl; 100 nm) or with siRNA against Cdk5 (siCdk5; 100 nm) for 3 d. Knockdown of Cdk5 (siCdk5-treated neurons) prevented cyclin B1 accumulation in glutamate-treated neurons. In A, C, D, and E, a representative Western blot is shown of three. Bar graphs represent the relative cyclin B1 protein abundance, as normalized with GAPDH, averaged from at least three independent neuronal cultures. In all cases, the represented values are means ± SEM (n = 3–4 independent neuronal cultures). *p < 0.05 versus untreated (−Glu) neurons. F, Glutamate triggered a time-dependent stimulation of Cdk1 activity, as assessed by the ability of the protein extracts to phosphorylate, in vitro, histone H1. Transfection with siCdk5 abrogated glutamate-caused Cdk1 activation. Each bar represents the mean ± SEM of three independent neuronal cultures. *p < 0.05 versus siControl 0 h. G, Neurons on day 4 in vitro were transfected with an shRNA against luciferase (Control) or with an shRNA against cyclin B1 (shCyclin B1) for 2 d. Then, neurons were incubated (or not in the Control condition) with glutamate (100 μm, 5 min; Glutamate condition) after incubation with culture medium for the indicated time points. When indicated, Rosc (10 μm) was added to the culture medium. Both shCyclin B1 and Rosc abrogated neuronal apoptotic death caused by glutamate but did not modify apoptosis in control (untreated) neurons (20 h: shCyclin B1, 13.52 ± 0.89%; Rosc, 12.77 ± 0.79%), as assessed by annexin V+/7-AAD− quantification by flow cytometry. Representative flow cytometric dot plots are shown of four independent experiments. Data are the mean ± SEM from four independent neuronal cultures (n = 4). *p < 0.05 versus control; #p < 0.05 versus glutamate. AD, Actinomycin D; MW, molecular weight.