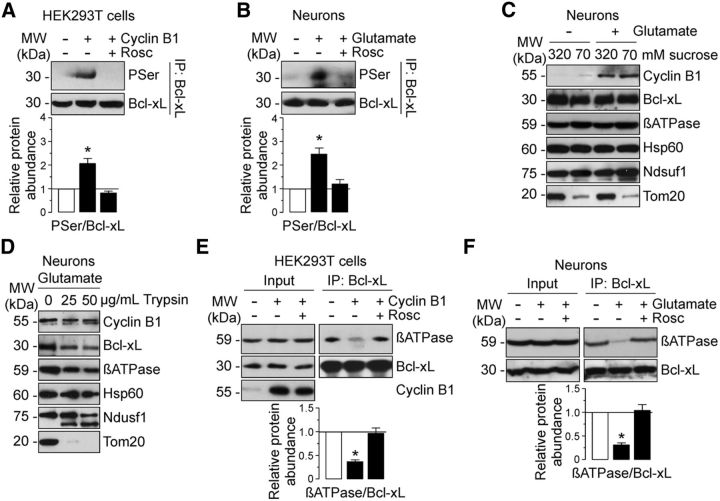
Figure 6.
cyclin B1–Cdk1 phosphorylates Bcl-xL, leading to its dissociation from β-F1Fo–ATP synthase on the excitotoxic stimulus. A, HEK293T cells were transfected with 0.8 μg/106 cells pIRES2–EGFP, either empty or containing the full-length cDNA of human cyclin B1, in either the absence of presence of Rosc (10 μm). At 24 h after transfections, cellular extracts were obtained and immunoprecipitated with anti-Bcl-xL antibody and analyzed by Western blot for phospho-Serine (PSer) and Bcl-xL. Expression levels of cyclin B1 (Input) is shown in Figure 5C. B, Neurons were treated or not with 100 μm glutamate for 5 min and were further incubated in culture medium for 20 h. When indicated, Rosc (10 μm) was added to the culture medium. Neuronal extracts were immunoprecipitated with anti-Bcl-xL antibody and analyzed by Western blot for phospho-Serine (PSer) and Bcl-xL. C, D, Neurons were exposed to glutamate as in B, and submitochondrial localization of cyclin B1, Bcl-xL, and β-F1Fo–ATP synthase was detected by mitoplasting (C) and protease protection assay (D). C, Mitochondria isolated from untreated (−Glu) or glutamate-treated (+Glu) neurons were incubated in isosmotic (320 mm) or hypotonic (70 mm) sucrose buffer, and extracts were subjected to Western blot analysis with cyclin B1, Bcl-xL, β-ATPase, Hsp60 (a matrix protein), Ndusf1 (and inner membrane protein), and Tom20 (an outer membrane protein). D, Mitochondria isolated from glutamate-treated neurons were incubated in mitochondrial buffer with or without 25 or 50 μg/ml soybean trypsin. Extracts were analyzed by immunoblotting with indicated antibodies. E, HEK293T cells were transfected as described in A. Cellular extracts were immunoprecipitated with anti-Bcl-xL antibody and analyzed by Western blot for β-F1Fo–ATP synthase (βATPase) and Bcl-xL. Of the whole cellular extracts used for immunoprecipitation, 10% were loaded on SDS-PAGE as an input control. Coimmunoprecipitation assays revealed that β-F1Fo–ATP synthase failed to coprecipitate with Bcl-xL in cyclin B1-transfected cells, which was prevented by Rosc. F, Neurons were exposed to glutamate as in B, and neuronal extracts were immunoprecipitated as in E. Coimmunoprecipitation assays revealed that glutamate avoided β-F1Fo–ATP synthase and Bcl-xL interaction in neurons, which was prevented by Rosc. In A, B, E, and F, a representative Western blot is shown of three. Bar graphs represent the relative band intensity compared with −Cyclin B1 (A, E) or −Glu (B, F). Each bar represents the mean ± SEM of three independent cell (A, E) or neuronal (B, F) cultures (n = 3). *p < 0.05 versus −Cyclin B1 (A, E) or − Glu (B, F). MW, Molecular weight.