Figure 4.
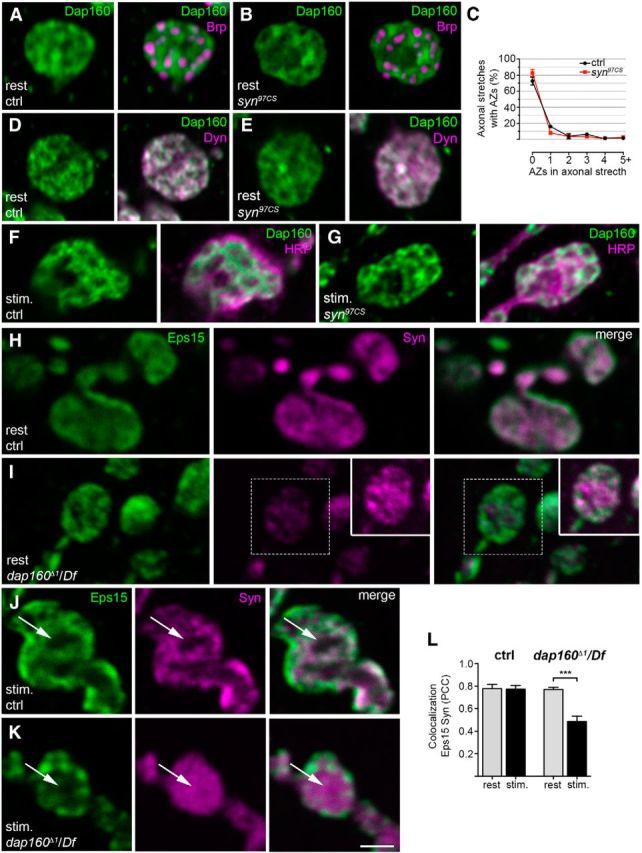
Dap160 is essential for the synaptic localization of synapsin (Syn), but deletion of syn does not alter Dap160 distribution in synapses. A, B, D–G, Confocal images of NMJs from control (ctrl) and synapsin-null mutant syn97CS at rest (A, B, D, E) and after stimulation with high K+ (F, G). A, B, Staining for Dap160 (green) and the T-bar protein Bruchpilot (Brp, magenta). C, Quantification of the percentage of active zones (AZs), as revealed by Brp labeling, in axonal stretches connecting boutons in control and syn97CS NMJs. Data are represented as mean + SEM; p > 0.05, one-way ANOVA with Bonferroni's post-test; n = 3. D, E, Staining for Dap160 (green) and its binding partner, the GTPase dynamin (Dyn, magenta). F, G, Images of NMJs from control and syn97CS stained with antibodies against Dap160 and HRP (magenta) to reveal bouton outline. H, I, Confocal images of middle optical sections of NMJs from the dap160-null mutant dap160Δ1/Df at rest stained with antibodies against the endocytic protein Eps15 (green) and Syn (magenta). The level of Syn immunoreactivity is decreased in NMJs from the dap160-null mutant compared with the control as previously shown (Koh et al., 2004), while the level of Eps15-immunoreactivity is not altered (Koh et al., 2007). Inset in I shows the Syn labeling from the same area at higher gain. J, K, Confocal images of middle optical sections from control and dap160Δ1/Df nerve terminals after high-K+ stimulation stained with the same antibodies. Following stimulation, the center of the bouton (arrows) is devoid of Syn labeling in control NMJs, whereas dap160Δ1/Df NMJs display dispersed Syn labeling. Scanning settings for the Syn channel is optimized as for the inset in I. L, Colocalization between Eps15 and Syn in control and dap160Δ1/Df NMJs at rest and during stimulation. Bars represent mean PCC + SEM; ***p < 0.001, Student's t test; n = 3–8. Scale bar: A, B, D, E–K, 2 μm.
