Figure 3.
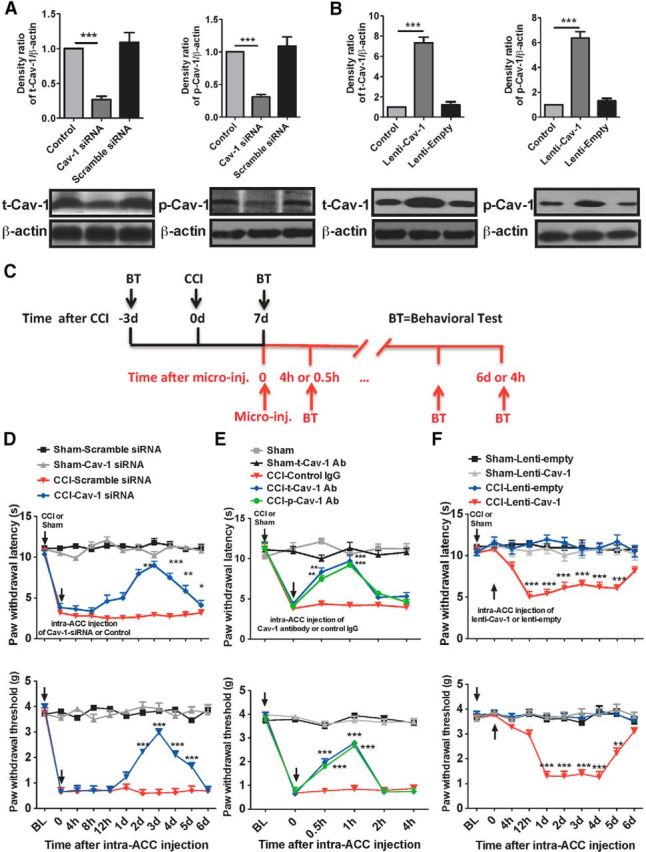
Manipulation of Cav-1 in the ACC modulates pain behavior. A, B, The expression of t-Cav-1 and p-Cav-1 by immunoblotting assay was used to validate efficient delivery tools of Cav-1 siRNAs (for knockdown of Cav-1) and lentivirus Cav-1 (Lenti-Cav-1; for overexpression of Cav-1) in vivo by intra-ACC injection. Microinjection of Cav-1 siRNAs (10 μg/0.5 μl) or Lenti-Cav-1 (0.5 × 106 TU/0.5 μl), not their controls, significantly inhibited or increased t-Cav-1 and p-Cav-1 expression in the ACC. The ACC samples were collected at 72 h after microinjection (n = 4). ***p < 0.001, compared with naive control or scramble siRNA or Lenti-empty control. C, Schematic illustration of experimental protocol. D, Microinjection of Cav-1 siRNAs (10 μg/0.5 μl), not their control scramble siRNAs, into contralateral ACC at 7 d after surgery significantly reversed CCI-induced thermal hyperalgesia and mechanical allodynia (n = 8). ***p < 0.001, **p < 0.01, and *p < 0.05, compared with CCI-scramble siRNA. E, Microinjection of anti-t-Cav-1 antibody (0.2 μg/0.5 μl) or anti-p-Cav-1 antibody (0.1 μg/0.5 μl), not its control IgG, into contralateral ACC at 7 d after surgery significantly reversed CCI-induced thermal hyperalgesia (top) and mechanical allodynia (bottom; n = 8). ***p < 0.001, **p < 0.01, compared with CCI-Control IgG. F, Microinjection of Lenti-Cav-1 (0.5 × 106 TU/0.5 μl), not its control Lenti-empty, into ipsilateral ACC at 7 d after surgery induced a significant decrease of thermal and mechanical pain threshold in the unaffected hindpaw (n = 8). ***p < 0.001, compared with CCI-Lenti-empty. BL, baseline.
