Figure 1.
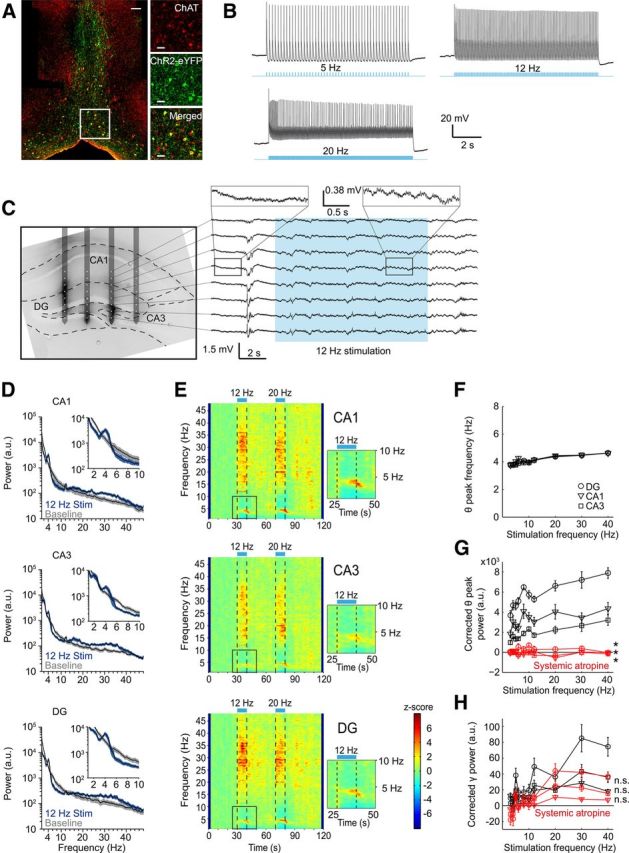
In vivo optogenetic activation of ChAT+ MSDB neurons control hippocampal rhythmogenesis. A, ChR2-eYFP is expressed in ChAT+ MSDB neurons after virus injection of a Cre-dependent rAAV into the MSDB of ChAT-Cre mice. Left, Lower-magnification photograph of ChAT-labeled (red) and ChR2-eYFP-labeled (green) cells of the medial septal region. Scale bar, 50 μm. Right, Higher magnification of the indicated area on the left. Scale bar, 20 μm. B, In vitro entrainment of a ChAT+-ChR2+ MSDB neuron evoked by repetitive 473 nm light stimulation with 20 ms light pulses. C, Left, Fifty-micrometer slice of the dorsal hippocampus overlaid with the schematic drawing of the in vivo electrode recording positions. Silicon probe shanks were coated with dextran-biotin for post hoc staining of shank-adjacent tissue. Right, Example trace of LFPs recorded from the eight electrode contacts of one silicon probe shank during 473 nm light stimulation at 12 Hz with 20 ms light pulses. D, Power spectra of a 10 s time interval shifted 2 s in relation to the period of stimulation at 12 Hz to account for the delayed on- and offset of stimulation-induced theta oscillations for hippocampal subfields CA1, CA3, and DG compared with power spectra of a 10 s baseline period. Black lines represent mean and blue and gray shading represent SEM of spectral power associated with stimulation-induced and baseline activity, respectively (n = 11 mice, data for one mouse reflect the mean across 3–6 trials). E, Time-frequency plots of power spectral density z-scores from LFPs recorded in hippocampal subfields CA1, CA3, and DG during light stimulation of ChAT+-ChR2+ MSDB neurons for 10 s intervals at 12 or 20 Hz. Insets show theta power increase and suppression of adjacent frequency bands during 12 Hz stimulation. F, Stimulation-induced theta peak power frequency in hippocampal subfields CA1, CA3, and DG increased linearly with frequency of stimulation (r = 0.88 for CA1, r = 0.97 for CA3, and r = 0.96 for DG, p < 0.001, r = Pearson's correlation coefficient). G, Stimulation-induced changes in corrected theta peak power in hippocampal subfields CA1, CA3, and DG increased linearly with stimulation frequencies (r = 0.68 for CA1, r = 0.92 for CA3, and r = 0.89 for DG, p < 0.05 for CA1, and p < 0.001 for CA3 and DG). Systemic atropine injections (50 mg/kg body weight) abolished theta induction in hippocampal subfields CA1, CA3, and DG (*p < 0.05, Wilcoxon signed-rank test). H, Stimulation-induced gamma band (26–48 Hz) power in hippocampal subfields CA1, CA3, and DG for different frequencies of light stimulation before and after systemic atropine injection (50 mg/kg body weight). n.s., Not significant, Wilcoxon signed-rank test or Student's paired t test. F–H, Symbols and error bars represent mean ± SEM for six mice with one value missing for CA1 at 40 Hz stimulation in F.
