Figure 9.
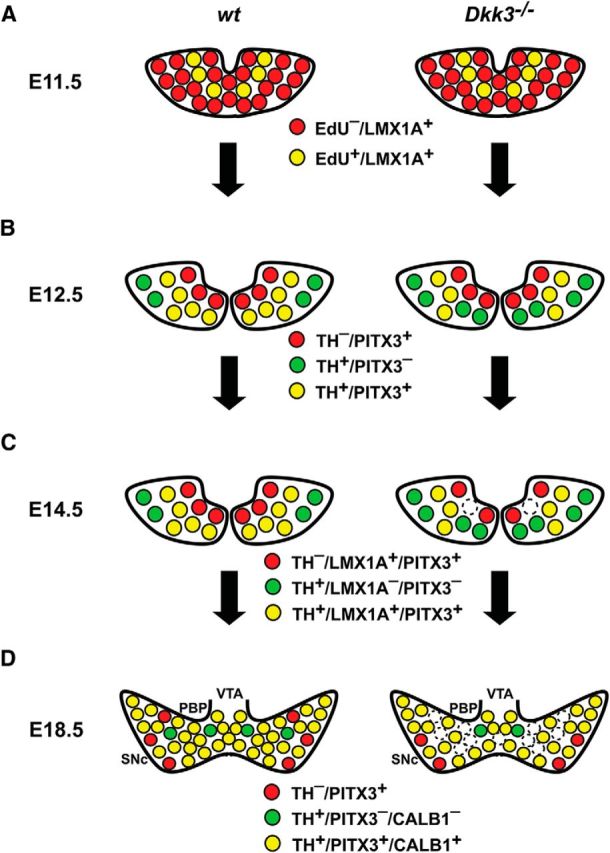
Selective differentiation defects and loss of a rostrolateral mdDA neuronal subset in Dkk3−/− mice. A–D, Schematic coronal views of the rostrolateral mdDA domain in the VM of E11.5 (A), E12.5 (B), E14.5 (C), and E18.5 (D) WT and Dkk3−/− embryos, depicting the phenotypic defects of the mutant embryos. A, Initially, at E11.5, the development of proliferating (EdU+) mdDA progenitors and postmitotic (EdU−) precursors expressing LMX1A (LMX1A+) proceeded normally in the Dkk3−/− embryos. B, At E12.5, the first defects became apparent in the mutant embryos: the numbers of TH+/PITX3− single-positive (green) cells were increased at the expense of TH+/PITX3+ double-positive (yellow) cells in the Dkk3−/− VM, whereas TH−/PITX3+ single-positive (red) cells remained unaffected at this stage. C, At E14.5, the numbers of TH+/LMX1A− or PITX3− single-positive (green) cells were still increased at the expense of TH+/LMX1A+ or PITX3+ double-positive (yellow) cells in the Dkk3−/− VM, but TH−/LMX1A+ or PITX3+ single-positive (red) cells were also decreased at this stage in the mutants. D, Shortly before birth, at E18.5, TH−/PITX3+ (red), TH+/PITX3−, or CALB1− (green), and TH+/PITX3+ or CALB1+ (yellow) single- and double-positive cells were reduced by at least one-fifth (∼20%) in the rostrolateral mdDA domain that comprises mostly the PBP and dorsomedial SNc. In vitro application of DKK3 protein rescued this selective differentiation defect in differentiating primary cells from the Dkk3−/− VM and promoted the differentiation of PSCs into TH+/PITX3+ double-positive mdDA neurons with molecular SNc DA characteristics (CALB1−/KCND3+/DAT+). Note that the coexpression of PITX3 and LMX1A or CALB1 in the same cell, as suggested by the scheme, has not been demonstrated in this work.
