Figure 9.
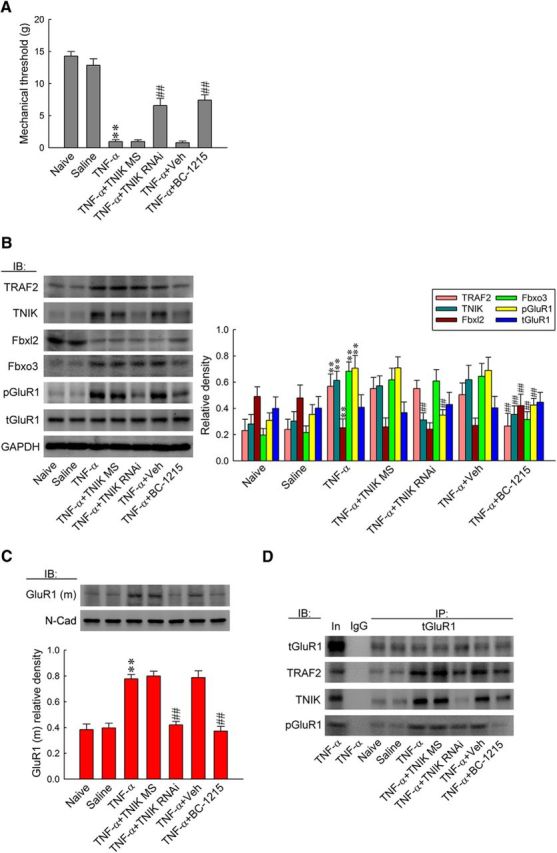
TNF-α induces allodynia via spinal Fbxo3/Fbxl2/TRAF2/TNIK/pGluR1-dependent GluR1–AMPAR trafficking. A, Statistical analysis of von Frey test showing intrathecal application of TNF-α (1 pm, 10 μl) significantly decreased the withdrawal threshold of the ipsilateral hindpaw at 2 h after injection, which was prevented by pretreatment with TNIK mRNA-targeting siRNA (5 μg, 10 μl, once daily for 4 d before TNF-α application) and BC-1215 (100 nm, 10 μl, 2 h before TNF-α application) but not by missense siRNA (5 μg, 10 μl) or the vehicle solution (10 μl). One-way ANOVA, post hoc Tukey's test: F(6,42) = 61.05, p < 0.001. **p < 0.01 versus Naive; ##p < 0.01 versus TNF-α. n = 7. B, C, Administration with TNIK mRNA-targeting siRNA prevented TNF-α-increased TNIK, pGluR1, and GluR1(m) expression but exhibited no effect on the levels of TRAF2, Fbxl2, Fbxo3, and tGluR1. Intrathecal pretreatment with BC-1215 prevented increases in TRAF2, TNIK, Fbxo3, pGluR1, and GluR1(m) expressions and decreases in Fbxl2 expression caused by TNF-α injection. However, it had no effect on tGluR1 expression. One-way ANOVA, post hoc Tukey's test: TRAF2, F(6,35) = 21.42, p < 0.001; TNIK, F(6,35) = 23.9, p < 0.001; Fbxl2, F(6,35) = 14.28, p < 0.001; Fbxo3, F(6,35) = 51.42, p < 0.001; pGluR1, F(6,35) = 37.03, p < 0.001; tGluR1, F(6,35) = 0.422, p = 0.859; GluR1(m), F(6,35) = 29.21, p < 0.001. **p < 0.01 versus Sham 7D; ##p < 0.01 versus SNL 7D. n = 6. D, Bolus of BC-1215 prevented TNF-α-enhanced tGluR1-TRAF2/TNIK/pGluR1 coprecipitation. Daily TNIK mRNA-targeting siRNA administration exhibited similar effects with the exception that it failed to affect TNF-α-enhanced tGluR1-TRAF2 coprecipitation.
