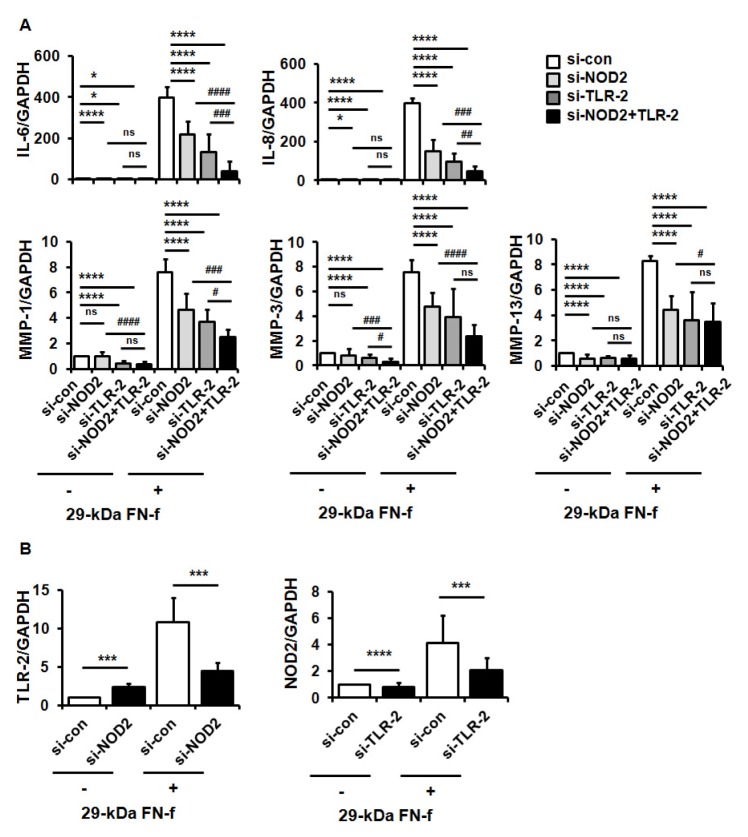
Fig. 2.
29-kDa FN-f increased pro-catabolic factor expressions through synergistic activation of NOD2 and TLR-2. (A) NOD2 and TLR-2 silencing inhibited 29-kDa FN-f-induced IL-6, IL-8, MMP-1, -3, and -13 expressions. Chondrocytes were transfected with control small interfering (si) RNA (si-con), si-NOD2 RNA (si-NOD2), or TLR-2 RNA (si-TLR-2), and 48 h later chondrocytes were treated with 29-kDa FN-f for 24 h. mRNA levels were measured using qPCR. Data represent the mean ± SD of duplicate data from more than five different donors. GAPDH served as an endogenous control. *P < 0.05 and ****P < 0.001 vs. si-contransfected cells. #P < 0.05, ##P < 0.01, ###P < 0.005, and ####P < 0.001 vs. 29-kDa FN-f+si-NOD2 and siTLR-2-transfected cells. ns, not significant. (B) The reciprocal regulation of NOD2 and TLR-2 on their mRNA expressions. mRNA expression of NOD2 and TLR-2 was measured in 29-kDa FN-f+si-NOD2- or 29-kDa FN-f+si-TLR-2-transfected chondrocytes using SYBR Green-based real-time PCR assay. GAPDH was used as an endogenous control. Data represent the mean ± SD of duplicate data from more than five different donors. ***P < 0.005 and ****P < 0.001 vs. si-NOD2- or si-TLR-2-transfected cells.