Figure 4.
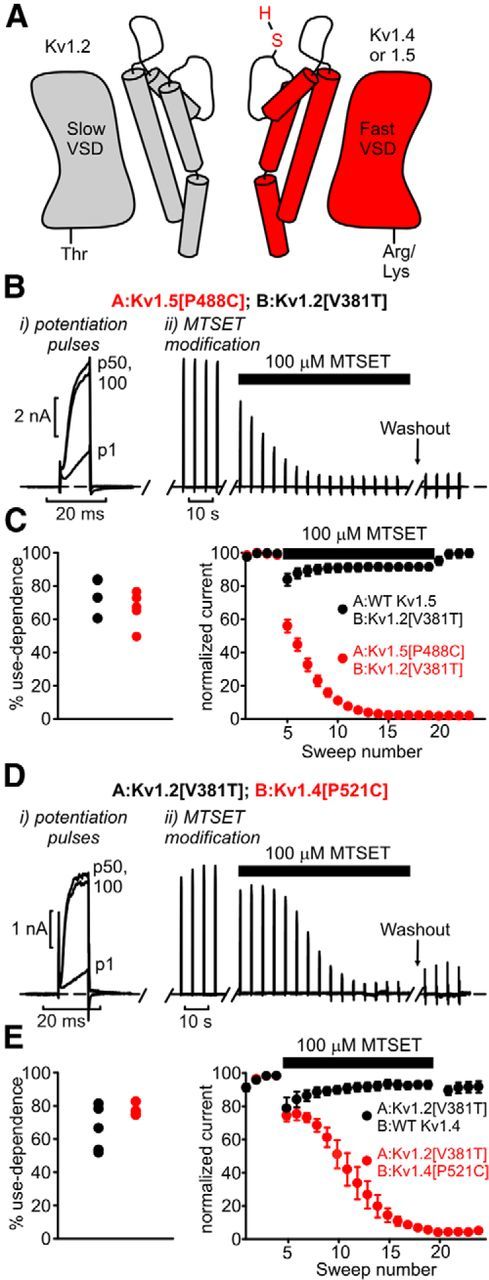
Functional contribution of Kv1.2 to heteromeric channel complexes that exhibit use-dependent activation. A, Dimeric channel constructs were generated comprising Kv1.2[V381T] and Kv1.5 (with or without a substituted cysteine at position P488) or Kv1.4 (cysteine substitution at P521). This design enables us to test whether channels that exhibit use-dependent activation (attributable to Kv1.2) also contain Kv1.5/Kv1.4 subunits in the functional channel. VSD indicates voltage-sensing domain. B, Cells transfected with A:Kv1.5[P488C]; B:Kv1.2[V381T] dimeric constructs were subjected to a series of 100 repetitive brief depolarizations (potentiation pulses of 10 ms, 60 mV, 20 Hz) to demonstrate use-dependent activation (Bi). Next, in Bii (MTSET modification), cells were exposed to 100 μm MTSET in the bath solution and pulsed to 60 mV for 400 ms, every 4 s. C, Mean data describing the experiments in B, with the distribution of percentage use-dependent activation for both constructs (left) and the MTSET sensitivity of both constructs (right). In dimers containing WT Kv1.5 (with no modifiable cysteines), no rundown is observed (black symbols). However, dimers comprising Kv1.5[P488C] exhibit virtually complete channel rundown, n = 4–5 per construct. D, E, Similar experiments as in B and C were performed using A:Kv1.2[V381T]; B:Kv1.4[P521C] dimeric channels, n = 4–5 per construct.
