Figure 6.
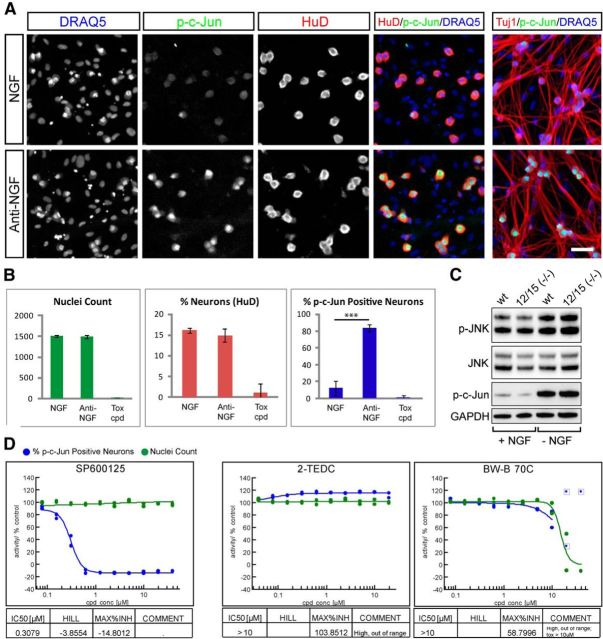
Lipoxygenases do not protect axons via alteration of JNK signaling. A, B, Measurement of p-c-Jun induction 2 h after NGF withdrawal in a 384-well setup. A, NGF and anti-NGF control wells are stained to reveal nuclei (DRAQ5), p-c-Jun, axons (Tuj1), and neuronal nuclei (HuD). Two hours after NGF withdrawal there is robust phosphorylation of c-Jun in neuronal nuclei without signs of axon degeneration. Scale bar, 50 μm. B, Quantification of example data shown in A. Anti-NGF treatment results in a significant increase in p-c-Jun-positive neurons (percentage p-cJun-positive neurons; NGF: 12.4 ± 3.9 and anti-NGF: 83.5 ± 4.0; ***p < 0001, Student's t test, error bars indicate SD) but does not alter nuclei count (NGF: 1511 ± 28, anti-NGF: 1495 ± 42) nor neuron content (percentage neurons; NGF: 16.1 ± 0.6%, anti-NGF: 14.9 ± 1.5%), while the toxic compound (tox cpd; 40 μm PD-146176) abolished all readouts (data from one experiment is shown, n = 7 for NGF and anti-NGF, n = 4 for PD-146176). C, Western blots measuring phosphorylation of JNK and c-Jun in Alox12/15(−/−) and wild-type DRG neurons in the presence of NGF (+ NGF) or 3 h after NGF withdrawal (− NGF). A similar induction of JNK pathway activity occurs in both genotypes. D, Dose–response curves showing effect of treatment with JNK inhibitor SP600125, 2-TEDC, or BW-B 70C on p-c-Jun-positive cell number. SP600125 resulted in p-c-Jun reduction with an IC50 of 0.31 μm while LOX inhibitors 2-TEDC and BW-B 70C had no effect on p-c-Jun.