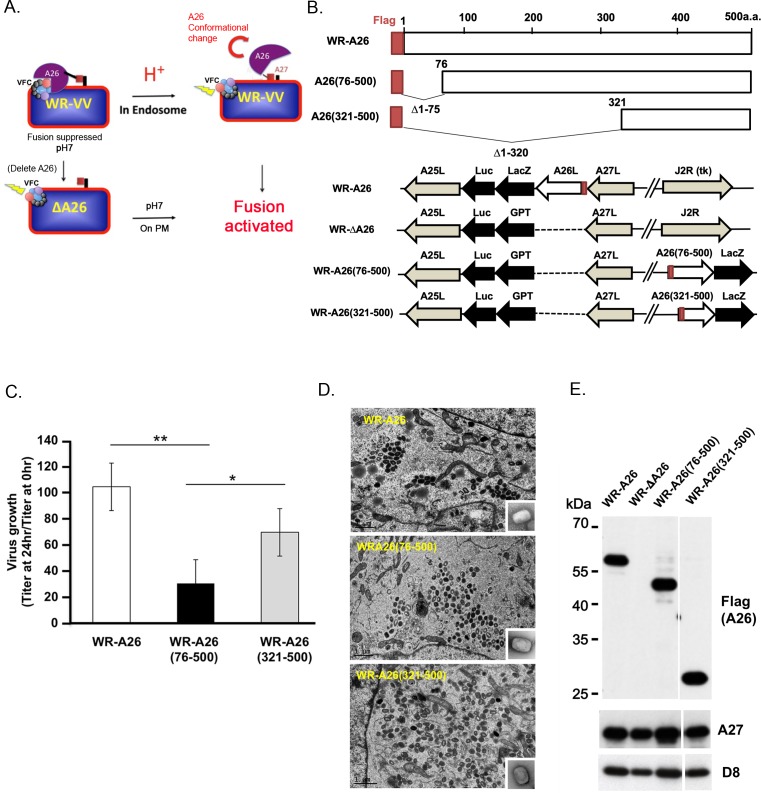
Fig 1. Model of A26 protein function and generation of mutant vaccinia viruses containing N-terminal deletions of A26 protein.
(A). Current model of how A26 protein functions as an acid-sensitive fusion suppressor of the WR strain of vaccinia virus MV. Vaccinia MV contains A26 protein that binds to the A16 and G9 components of the viral fusion complex (VFC) to suppress virus membrane fusion activity, which keeps the virus stable at neutral pH. When MV is internalized into endosomes, acidification triggers conformational changes of A26 protein that dissociates itself from the VFC, resulting in activation of the VFC and fusion of the viral and vesicular membranes. However, deletion of the A26L gene results in WR-ΔA26 MV particles lacking fusion suppressor activity, so these MV particles readily fuse to the plasma membrane (PM) at neutral pH. (B). Schematics of full-length WR-A26 and truncated WR-A26 proteins (aa 76–500 or 321–500), as well as each construct containing flag-tag sequences (red) at their N-termini. Locations of A26L mutant genes in the corresponding viral genomes are also shown. WR-A26 and WR-ΔA26 were described previously [32, 33], and the latter was used as the parental virus to generate the WR-A26(76–500) and WR-A26(321–500) recombinant viruses. The J2R locus encodes a non-essential viral thymidine kinase (tk). We inserted A26(76–500) and A26(321–500) gene constructs into the tk locus and then selected blue plaques in agar plates containing X-gal. (C). One-step growth of mutant vaccinia viruses in HeLa cells. Cells were infected with each virus at a multiplicity of infection (MOI) of 5 PFU per cell and cells were washed and harvested at 0 and 24 hpi for virus titer determination. The Y-axis represents MV growth, determined by dividing virus titers at 24 hpi with the respective input virus titer at 0 hpi. We performed three experimental repeats for each virus and used the Student t-test for statistical analyses. *p <0.05, ** p < 0.01. (D). EM images of MV in infected HeLa cells at 24 hpi. Insets in lower right corner represent EM images of CsCl-purified MV. The scale bar represents 1 μm. (E). Immunoblots of N-terminal A26-deletion proteins in CsCl-purified MV particles. A27 and D8 are two viral envelope proteins that served as positive controls.