Fig 8. Vaccinia virus membrane protein A26 is a fusion suppressor of MV.
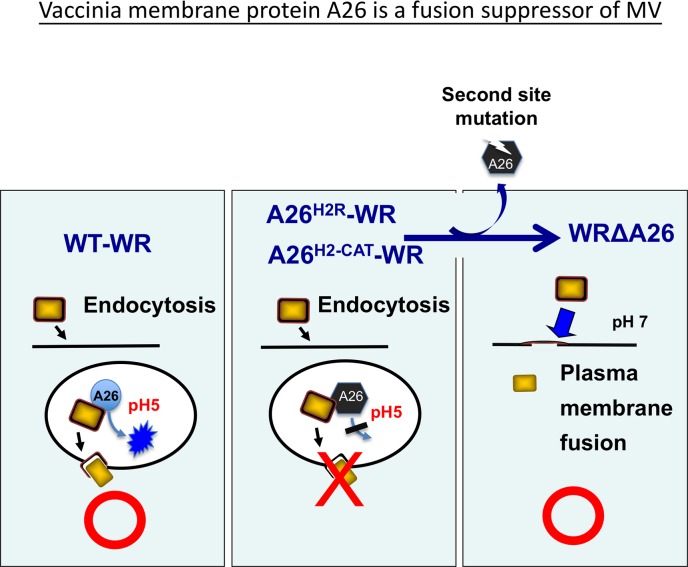
Data presented in this study reveal an important role for A26 protein in endocytic entry of the WR strain of vaccinia MV. Low pH in endosomes triggers conformational changes of wild type A26 protein to allow membrane fusion within vesicles. However, when mutant A26 protein, such as A26H2R or A26H2-CAT, loses the ability to execute conformational changes at low pH, it becomes a constitutive suppressor that blocks virus membrane fusion, resulting in significant loss of mature virus infectivity. Subsequent generation of the second-site mutations then leads to truncation of A26 protein so the resulting revertant viruses acquire a phenotype similar to that of WRΔA26 virus and initiate virus fusion with the plasma membrane at neutral pH to recover MV infectivity.