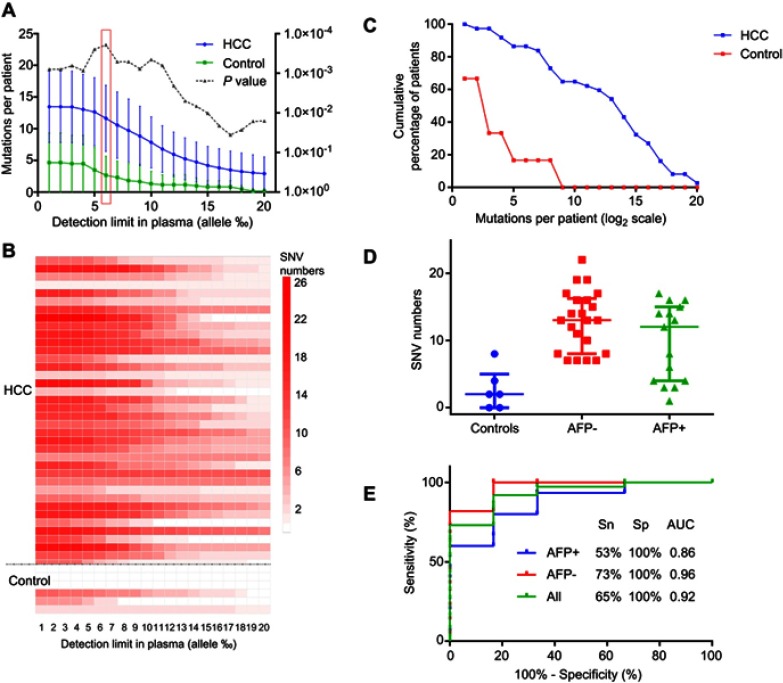
Figure 2.
Detection of the somatic mutations in plasma cfDNA and their performance for the diagnosis of HCC. The selected somatic mutations were examined in plasma cfDNA samples derived from HCC patients (n=37) and healthy individuals (n=6) as controls. (A–C) The numbers of mutations per patient detected in the plasma cfDNA samples in the HCC and control groups. The numbers of mutations reached a peak value at the detection limit of 0.6% (allele), and this was considered as a cutoff value in the subsequent analysis. (D) Comparison of the mutation numbers detected in the AFP-positive group and AFP-negative group with the cutoff value of 0.6% (allele). Based upon the status of AFP, the 37 HCC patients were classified into two subgroups: AFP-negative group (AFP <400, n=22) and AFP-positive group (AFP ≥400, n=15), (E) ROC analysis of the mutations in the HCC and control plasma cfDNA samples. The sensitivity and specificity at the cutoff value of 0.6% were calculated. The AUC was 0.92, with a sensitivity (Sn) of 65% and specificity of 100% for the diagnosis of HCC. The AUC was 0.96 with a sensitivity of 73% and specificity of 100% for HCC patients who were negative for AFP. The AUC value was 0.86 with a sensitivity of 53% and specificity of 100% for HCC patients who were positive for AFP.
Abbreviation: HCC, hepatocellular carcinoma.