Figure 3.
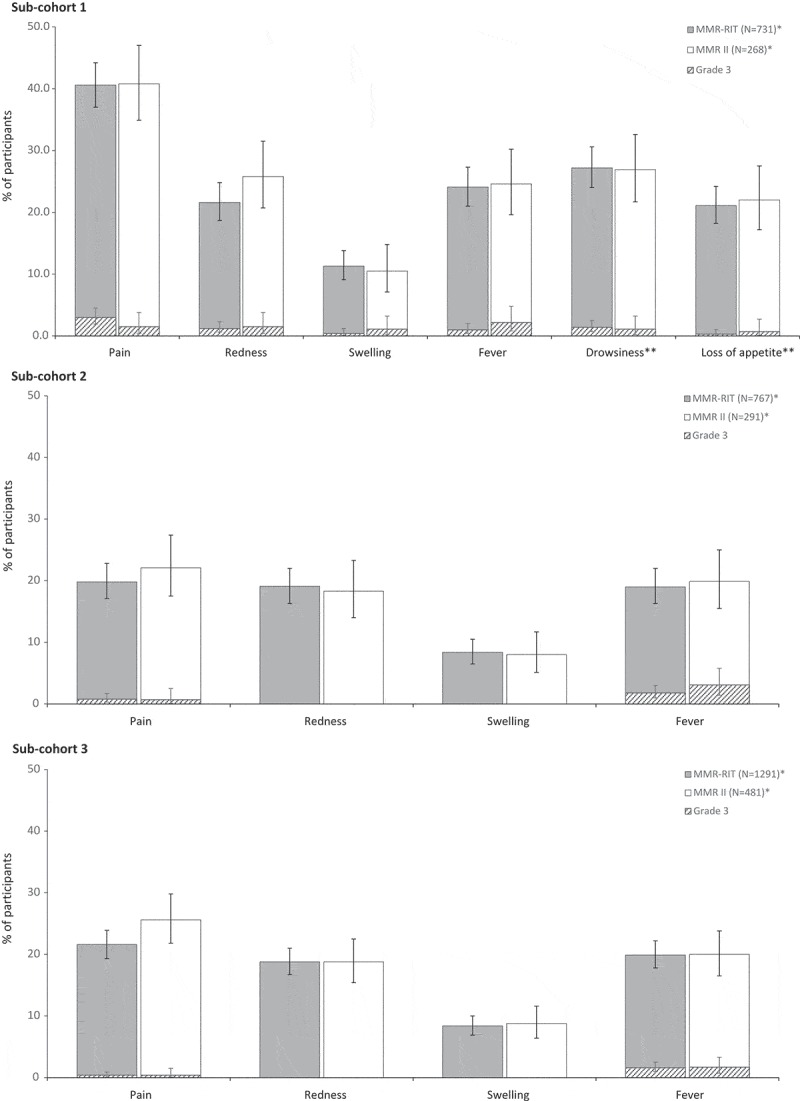
Incidence, in each sub-cohort, of solicited injection site adverse events (Day 0–3), fever (Day 0–42), and drowsiness and loss of appetite (Day 0–3; only assessed in sub-cohort 1) (total vaccinated cohort).
Footnote: N, number of participants with at least 1 vaccine administration documented.*Except for fever, drowsiness, and loss of appetite in sub-cohort 1 (MMR-RIT, N = 731 and MMR II, N = 268); fever in sub-cohort 2 (MMR-RIT, N = 767 and MMR II, N = 291); and fever in sub-cohort 3 (MMR-RIT, N = 1291 and MMR II, N = 481). Children in sub-cohort 1 received either MMR-RIT or MMR II together with DTaP-IPV and VV; children in sub-cohorts 2 and 3 received either MMR-RIT or MMR II alone.The injection site adverse events (i.e., pain, redness, and swelling) refer to the site of MMR vaccine injection. Fever: temperature ≥38.0°C. Grade 3 was defined as: limb spontaneously painful or child cried when limb was moved (pain); diameter >50 mm (redness, swelling); temperature >39.5°C (fever); adverse event preventing normal, everyday activities (drowsiness); not eating at all (loss of appetite). The error bars represent the upper and lower limits of the exact two-sided 95% confidence intervals.
