Figure 4.
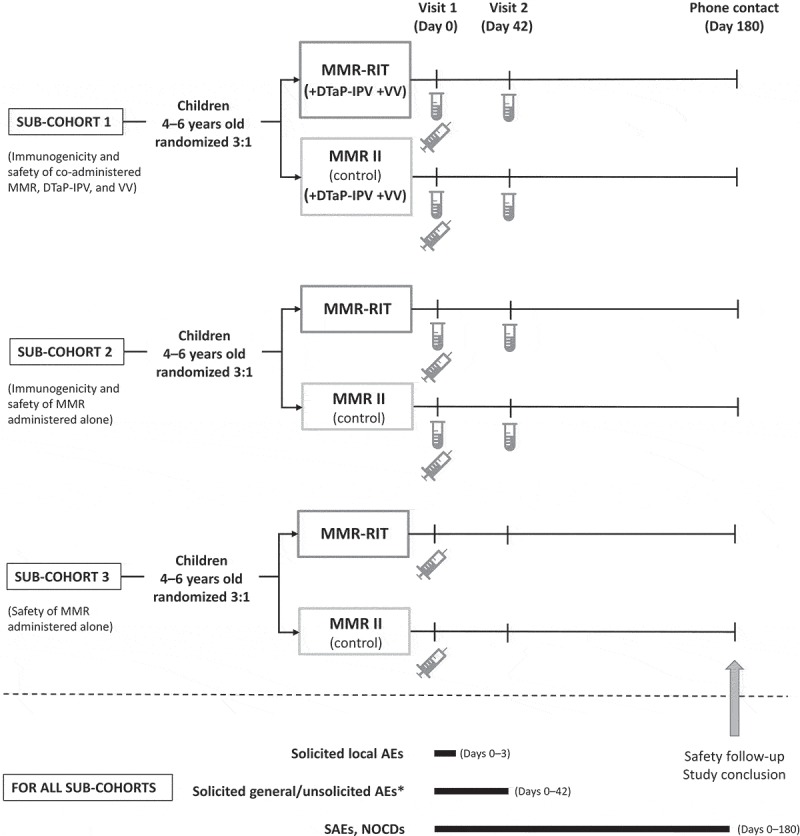
Study design.
Footnote:  , blood sampling;
, blood sampling;  , vaccine administration (includes administration of DTaP-IPV and VV in sub-cohort 1); AEs, adverse events; DTaP-IPV, diphtheria, tetanus, acellular pertussis, and inactivated polio vaccine; NOCDs, new onset chronic diseases; SAEs, serious adverse events; VV, varicella vaccine.*Drowsiness and loss of appetite were recorded as solicited general AE from Day 0 to Day 3 in sub-cohort 1 only.
, vaccine administration (includes administration of DTaP-IPV and VV in sub-cohort 1); AEs, adverse events; DTaP-IPV, diphtheria, tetanus, acellular pertussis, and inactivated polio vaccine; NOCDs, new onset chronic diseases; SAEs, serious adverse events; VV, varicella vaccine.*Drowsiness and loss of appetite were recorded as solicited general AE from Day 0 to Day 3 in sub-cohort 1 only.
