Figure 1.
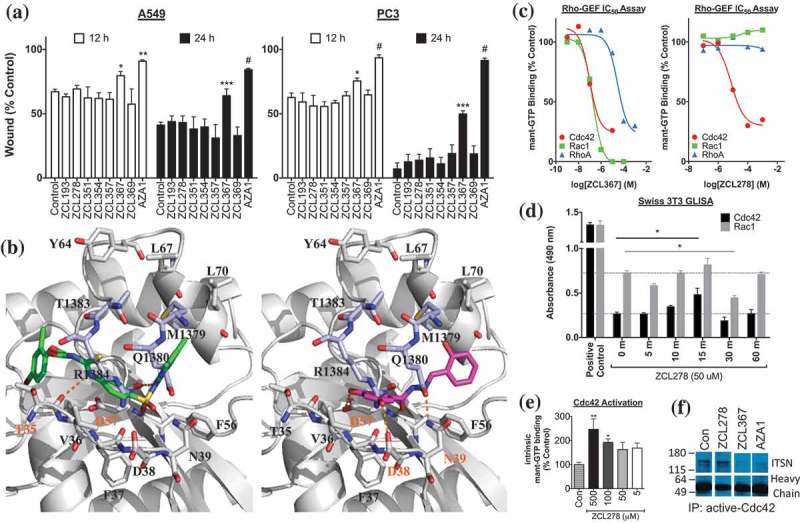
ZCL compound screening and validation as Cdc42-ITSN regulators. (a) ZCL367 inhibits migration/wound healing of A549 and PC3 cells. Confluent cells were scratched and treated with mitomycin C before treating with ZCL compound. The percentage of the wound closure was quantified from three scratches from two independent experiments and is expressed as mean±SEM. (b) Molecular docking of ZCL278 (green) and ZCL367 (pink) into the Cdc42-ITSN binding site. ZCL278 and ZCL367 show different poses with a certain extent of overlap with each other. ZCL278 formed two hydrogen bonds with Thr35 and Asp57, and hydrophobic interactions with Val36 and Thr35 of Cdc42. ZCL367 formed three hydrogen bonds with Asp38, Asn39, and Asp57, and hydrophobic interactions with Phe56 and Val36 of Cdc42. Gray: Cdc42, light purple: ITSN, orange dots: hydrogen bond. (c) ZCL compounds inhibit GEF-mediated Rho GTPase activation. Both ZCL278 (IC50 = 7.5 μM) and ZCL367 (IC50 = 0.098 μM) inhibit DH domain-mediated mant-GTP binding/Cdc42 activation. ZCL367 is more potent against Cdc42 than RhoA (IC50 = 29.7 μM) and Rac1 (IC50 = 0.19 μM). (d) ZCL278 activates Cdc42. Serum-starved Swiss 3T3 cells were treated with ZCL278 and analyzed for Cdc42 and Rac1 activation via GLISA. (e) ZCL278 increases intrinsic GTP binding of Cdc42. In the absence of GEF/DH domain, treatment with ZCL278 increased binding of GTP to Cdc42. (f) Cdc42 regulators inhibit Cdc42-ITSN. A co-immunoprecipitation of ITSN with active-Cdc42 revealed that ZCL367 (50 μM) and AZA1 (10 μM) was able to dislodge ITSN from active-Cdc42. At the same concentration, ZCL278 was not effective. All data are presented as mean±SEM from duplicates or triplicates from two independent experiments. ANOVA compared treatments to their respective control (* p < 0.05, ** p < 0.01, *** p < 0.001, # p < 0.0001).
