Abstract
Changes in physiological arousal frequently accompany cognitive and affective challenges. Many studies employed cue exposure paradigms to investigate the neural processes underlying cue-elicited drug and alcohol craving. However, whether cue-elicited craving relates to changes in physiological arousal and the neural bases underlying the potential relationship remain unclear. Here we examined cerebral cue-related activations in relation to differences in skin conductance responses (SCR) recorded during alcohol vs. neutral cue blocks in 61 non-dependent alcohol drinkers (30 men). Imaging and skin conductance data were collected and processed with published routines. Mediation analyses were conducted to examine the inter-relationship between regional activities, cue-elicited craving, and SCR. The results showed higher SCR during alcohol than during neutral cue exposure. Despite no differences in drinking characteristics, men as compared to women demonstrated higher craving rating, and men but not women demonstrated a positive correlation between alcohol (vs. neutral) cue-evoked craving and SCR. Further, across subjects, thalamic cue activity was positively correlated with differences in SCR between alcohol and neutral cue blocks in men but not in women. Mediation analyses suggested that thalamic activity mediated the correlation between craving and SCR across men and women, and in men but not women alone. These findings substantiate physiological and neural correlates of alcohol cue response and suggest important sex differences in the physiological and neural processes of cue evoked craving. Centered on the intralaminar and mediodorsal subregions, the thalamic correlate may represent a neural target for behavioral or pharmacological therapy to decrease cue-elicited arousal and craving.
Keywords: alcohol, thalamus, cue, electrodermal, galvanic skin response, arousal
Introduction
Skin conductance response (SCR) is a physiological index of arousal. Via sympathetic innervations of the skin sweat glands, heightened arousal is associated with increases in electrodermal conductance (Critchley, 2002; Naqvi and Bechara, 2006). The SCR can be elicited by effort, environmental stimuli, and/or emotional responses.
Craving for abused substances is known to be associated with changes in interoceptive responses and physiological arousal (Buckner et al., 2016; Carter et al., 2009; Childress et al., 1988; Fox et al., 2012; Li et al., 2015; Pachas et al., 2015; Robbins et al., 2000; Sinha et al., 2009; Trotzke et al., 2014; Zhao et al., 2012). Many studies have employed functional brain imaging to investigate the neural correlates of craving (see (Jasinska et al., 2014) for a review) and those underlying changes in physiological arousal in response to cognitive and affective challenges (Critchley et al., 2011). For instance, compared to controls, individuals with alcohol use disorders demonstrated greater alcohol cue-related activation of parietal and temporal regions, including the posterior cingulate, precuneus and superior temporal gyrus (Schacht et al., 2013). We recently demonstrated higher responses to alcohol vs. neutral cue responses in the occipital, retrosplenial, and medial orbitofrontal cortex as well as left caudate head in non-dependent drinkers (Zhornitsky et al., 2018). Changes in physiological arousal engages a wide swath of cortical and subcortical regions, including the insula and medial prefrontal cortex (Critchley, 2002). In particular, the ventromedial prefrontal cortex appears to play a specific role in regulating physiological arousal (Nagai et al., 2004; Zhang et al., 2013). On the other hand, no imaging studies have examined the neural processes underpinning cue-elicited changes in arousal, whether the regional responses correspond to cue-elicited activities, or how these neural activities relate to cue-elicited craving.
Women and men exhibited differences in cue evoked changes in craving, physiological and neuroendocrine responses, and other neurobiological processes that underlie the development of alcohol addiction (Barker and Taylor, 2017; Ceylan-Isik et al., 2010). Alcohol-associated stimuli induced craving in male but not female social drinkers (Willner et al., 1998). Male as compared to female binge drinkers showed more significantly elevated cue reactivity in event-related potentials (Petit et al., 2013). In social drinking males but not females, craving was associated with alcohol cue-induced striatal activation (Seo et al., 2011). A more recent study showed that, while inducing similar levels of self-reported craving, exposure to visual alcohol cues led to significantly stronger striatal activation in male compared to female drinkers (Kaag et al., 2018). On the other hand, an earlier work suggested no sex differences in cue reactivity in dependent alcohol drinkers (Rubonis et al., 1994). Together, these studies suggest the importance to consider sex differences in study of alcohol cue reactivity.
Here, sixty-one non-dependent drinkers participated in clinical assessments and an fMRI study of alcohol cue reactivity in conjunction with recordings of skin conductance activity. We addressed several issues. First, is alcohol cue-elicited subjective craving related to changes in physiological arousal? Second, what are the neural processes underlying cue-elicited changes in physiological arousal? Third, do male and female drinkers differ in cue-elicited changes in arousal and in the neural processes underlying the changes in arousal?
Materials and methods
Subjects, assessments and behavioral tasks
Candidates were recruited from the greater New Haven, CT area. Sixty-one non-dependent adult drinkers met eligibility and participated in this study, including 51 of the 61 participants (Zhornitsky et al., 2018) who were scanned with concurrent recording of skin conductance, and 10 new participants (Table 1). All subjects were required to be physically healthy with no major medical conditions. Those with current use of prescription medications or with a history of head injury or neurological illness were excluded. Other exclusion criteria included current or past dependence on a psychoactive substance (except nicotine) and current or history of Axis I disorders according to the Structured Clinical Interview for DSM-IV (SCID) (First, 1995). Candidates who reported current use of illicit substances or tested positive for cocaine, methamphetamine, opioids, marijuana, barbiturates, or benzodiazepines were not invited to participate. The Human Investigation committee at Yale University School of Medicine approved the study procedures. All participants signed an informed consent prior to the study.
Table 1:
Demographics and drinking measures of male and female participants
| Subject characteristic | Men (n=30) | Women (n=31) | P value* |
|---|---|---|---|
| Age (yrs) | 32.8 ± 11.6 | 35.7 ± 14.2 | 0.39 |
| AUDIT score | 10.0 ± 10.6 | 8.7 ± 9.3 | 0.61 |
| Duration of alcohol use (yrs) | 14.4 ± 11.3 | 17.0 ± 14.7 | 0.36 |
| # of drinking days/mo, prior yr | 8.4 ± 5.9 | 9.0 ± 5.6 | 0.67 |
| # of drinks/per occasion | 3.5 ± 2.5 | 3.1 ± 2.3 | 0.51 |
| # of drinks/mo, prior yr | 34.1 ± 43.0 | 32.8 ± 40.4 | 0.90 |
| GP score | 12.7 ± 5.7 | 13.5 ± 6.2 | 0.64 |
| FTND score | 0.4 ±1.5 | 1.0 ± 2.3 | 0.22 |
| Current smoker (yes/no) | 4/25§ | 10/21 | 0.09 |
Note: values are mean ± S.D.
two-tailed two-sample t test, except for smoker status which was based on Chi-square test; AUDIT: Alcohol Use Disorder Identification Test; GP: global positive subscore of the Alcohol Expectancy Questionnaire-3; FTND: Fagerström Test for Nicotine Dependence.
One subject’s data missing.
Participants were evaluated with Alcohol Use Disorders Identification Test (AUDIT) (Babor et al., 2001), an instrument widely used to assess alcohol use behavior and related problems. Participants were also evaluated with the Alcohol Expectancy Questionnaire or AEQ-3 (George et al., 1995). The AEQ-3 consists of 40 items to assess both positive (6 subscales) and negative (2 subscales) alcohol expectancy. Although the expectancy subcomponents are statistically discernible, the subscales are highly inter-correlated (George et al., 1995). Here, we used the global positive (GP) subscale (five items, with score from 5 to 30) to reflect positive alcohol expectancy. Participants were also assessed with the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al., 1991) and averaged 0.7 ± 1.9 (mean ± SD) in FTND score, suggesting low dependence.
Behavioral tasks
We employed a cue-induced alcohol craving task in the current study. Participants viewed alcohol-related or neutral pictures and reported alcohol craving in alternating blocks. Briefly, a cross was used to engage attention at the beginning of each block. After 2 s, six pictures displaying alcohol related cues (alcohol block) or neutral visual scenes (neutral block) were shown for 6 s each. Participants were asked to view the pictures and ponder how they might relate to the scenes. The pictures were collected from the Internet and independently reviewed by two investigators. Alcohol pictures included bar scenes, individuals or a group of people (both men and women) holding or drinking alcoholic beverages, and images of a variety of alcoholic drinks, such as beer, wine, and vodka. Neutral pictures comprised natural sceneries. Participants were asked at the end of each block to report how much they craved for alcohol from 0 (no craving) to 10 (highest craving ever experienced) on a visual analog scale. Each block lasted about 45 s, including time for craving rating. A total of 6 alcohol and 6 neutral blocks took approximately 9 m to complete. Each participant completed two runs of the task.
Skin conductance acquisition and analysis
We recorded skin conductance continuously during fMRI from the palmer surfaces of the index and middle fingers of the left hand with a Biopac MP150 system. The system employed a AcqKnowledge 4.1 software (Biopac Systems, USA) and the electrodermal activity amplifier module (Galvanic Skin Response [GSR] 100c) set at a channel sampling rate of 31 Hz and a gain of 5 μSiemens (μS) per volt (0.0015 μS resolution). Recording was synchronized with behavioral task and image acquisition. We applied a smoothing function (moving average of 500 ms) to eliminate high-frequency noise (Figner and Murphy, 2011), and computed the skin conductance response (SCR) to alcohol and neutral cues by subtracting the level of skin conductance over the entire 36 s (6 picture cues each presented for 6 s) from the baseline defined as the signal at time=0 s (Figner and Murphy, 2011; Gamer et al., 2007; Schiller et al., 2008; Spoormaker et al., 2011).
Imaging protocol and data analyses
Imaging protocol and data pre-processing followed publishd routines, as described in the Supplement.
In modeling of imaging data, we distinguished alcohol and neutral cue blocks for each individual subject using a general linear model (GLM) that included the realignment parameters in all six dimensions. We corrected for serial autocorrelation caused by aliased cardiovascular and respiratory effects by a first-degree autoregressive model. The GLM was used to estimate the component of variance explained by each of the regressors. We constructed for individual subjects a contrast of alcohol vs. neutral blocks to evaluate regional activities that differentiated viewing of alcohol and neutral pictures. We used the contrast or con (difference in β) images of the subject-level analysis for group-level statistical (random-effects) analyses. We performed a one-sample t test to identify regional response to alcohol vs. neural cues across subjects. We also performed a linear regression of con images against the differences in SCR to alcohol vs. neutral cues across subjects, with age as a covariate. Following current reporting standards, we evaluated the results with voxel p<0.001, uncorrected, in combination with cluster p<0.05, FWE corrected (Poldrack et al., 2017), on the basis of Gaussian random field theory, as implemented in SPM. For clusters comprising many distinct regions, we employed a higher threshold (p<0.0001, uncorrected or p<0.05 FWE corrected) to distinguish the individual peaks. In region of interest (ROI) analysis, we used MarsBaR (http://marsbar.sourceforge.net/) to derive for each individual subject the β contrast or cue-elicited activity for the ROIs. We defined functional ROIs from clusters of whole brain analysis, and showed all voxel activations in the Montreal Neurological Institute (MNI) coordinates.
Mediation analyses
We performed mediation analyses as described in the Supplement.
Results
Cue-elicited craving
Subjects reported higher craving during alcohol (3.0 ± 2.4) as compared to neutral (1.9 ± 2.0) cue blocks (t=7.13, p<0.001, paired sample t test). Men as compared to women showed greater difference in subjective rating of alcohol (vs. neutral) cue evoked craving (men: 1.5 ± 1.4; women: 0.8 ± 0.9; t=2.22, p=0.0300, two-sample t test).
Skin conductance responses
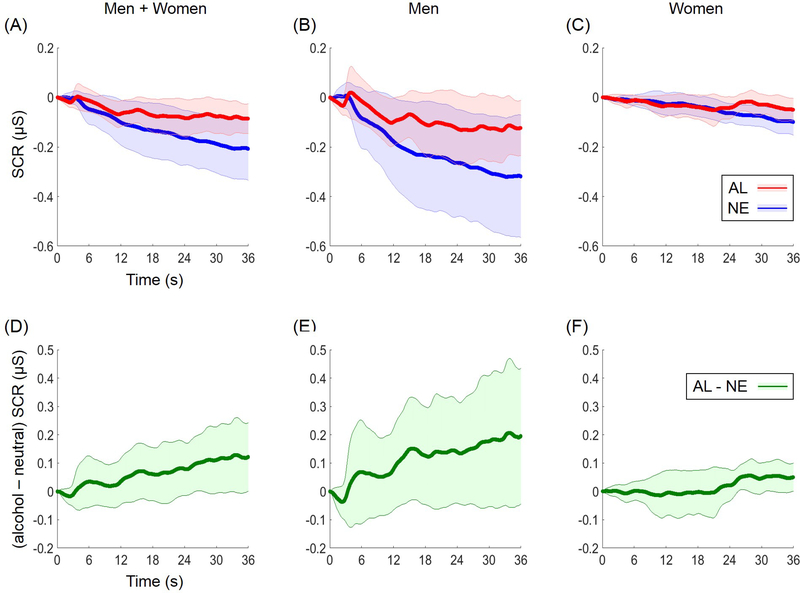
Skin conductance responses during alcohol and neutral cue blocks are shown in Figure 1. The SCR maintained a similar level during alcohol cue but gradually decreased over the neutral cue blocks. We compared the SCR between blocks for the entire 36 s. The difference in SCR between alcohol and neutral cue blocks was significant across participants (t=2.16, p=0.0349, two-tailed paired sample t test). Although the mean of SCR difference was numerically higher in men (0.1219 ± 0.3905 μS) than women (0.0406 ± 0.1386 μS), a direct comparison failed to demonstrate differences in men vs. women (t=1.09, p=0.2804; two-tailed two-sample t test).
Figure 1.
Skin conduct response (SCR) to alcohol (AL) and neutral (NE) cues. (A-C) The mean (line) and 95% confidence interval (shade) of alcohol (red) and neutral (blue) cue evoked SCRs. (D-F) The mean and 95% confidence interval of the difference in SCR (green): “alcohol minus neutral.” The data are shown for men and women combined (A, D), men (B, E), and women (C, F).
We also examined the correlation of SCR (alcohol – neutral) and cue-elicited craving (alcohol – neutral) across subjects. Linear regression showed a positive correlation in men and women combined (r=0.36, p=0.0043) and in men (r=0.41, p=0.0231), but not in women (r=0.01, p=0.9753) alone.
Across subjects, the AUDIT score was not correlated with cue-induced craving in men and women combined (r=0.02, p=0.8585), or in men (r=−0.15, p=0.4277) or women (r=0.27, p=0.1418) alone. The AUDIT score was also not correlated with differences in SCR between the alcohol and neutral cue blocks in men and women combined (r=−0.03, p=0.8006), in men (r=−0.09, p=0.6395) or in women (r=0.09, p=0.6410) alone.
GP score was positively correlated with the difference in craving rating between alcohol and neutral cue blocks for men and women combined (r=0.33, p=0.0090), for men (r=0.44, p=0.0160), but not for women (r=0.29, p=0.1081). GP score was not correlated with SCR (alcohol - neutral) in men and women combined (r=0.09, p=0.4829), or in men (r=0.08, r=0.6758) or women (r=0.20, p=0.2745) separately.
Regional responses to alcohol vs. neutral cues
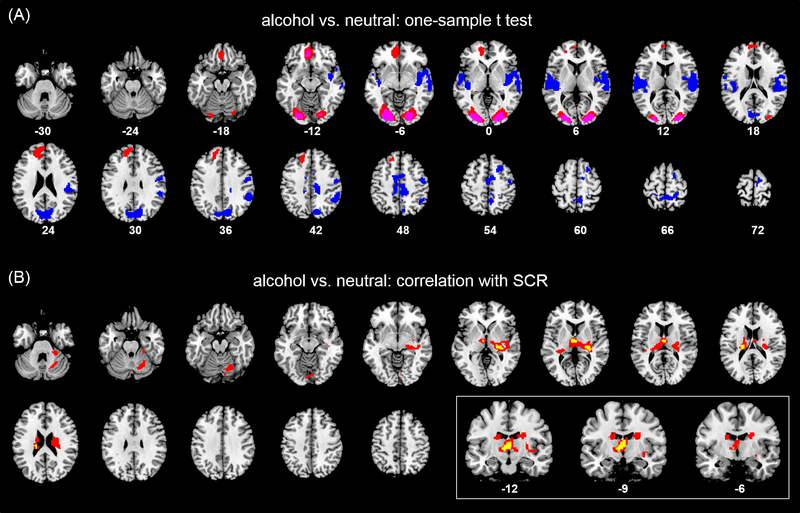
We first conducted a two-sample t test both with and without age as a covariate and observed that men and women did not show significant differences in regional responses to alcohol vs. neutral cues, at p<0.001, uncorrected. Thus, we combined men and women in examining cue-elicited regional responses. One-sample t test showed higher activities in response to alcohol vs. neutral cues in bilateral occipital cortices and bilateral but predominantly left-hemispheric ventromedial prefrontal and frontopolar cortices. Neural as compared to alcohol cues engaged higher activity in the cuneus/precuneus, mid-cingulate cortex/supplementary motor area, right inferior parietal cortex, and bilateral middle/superior temporal gyrus that involved the posterior insula and superior parietal lobule (Figure 2A). Regions meeting cluster p<0.05 FWE are summarized in Table 2.
Figure 2.
(A) Regional activations to alcohol vs. neutral cues (one sample t-test). Red and blue color indicates clusters obtained of alcohol > neutral and neutral > alcohol, respectively, at voxel p = 0.001, uncorrected. Pink color shows clusters with a voxel peak meeting p<0.05, FWE-corrected, for the contrast alcohol > neutral. (B) Regional activations to alcohol vs. neutral cues in positive correlation with SCR (alcohol – neutral) in a linear regression, shown in the same axial sections. Clusters in red were obtained with voxel p < 0.001, uncorrected and those in yellow with voxel p < 0.0001, uncorrected. We used the latter, more stringent threshold in order to better visualize the locations of peak activities. Neurological orientation: right = right. The three coronal sections in the inset highlight the intralaminar and mediodorsal thalamus.
Table 2.
Regional activations to alcohol vs. neutral cues, one-sample t-test.
| Region | Cluster | Voxel | MNI Coordinates (mm) |
||
|---|---|---|---|---|---|
| Size (voxels) | Z Value | X | Y | Z | |
| L OC* | 288 | 6.64 | −18 | −94 | 4 |
| R OC* | 245 | 6.30 | 21 | −91 | 4 |
| R vmPFC/FPC* | 40 | 5.20 | −6 | 44 | −11 |
| R Cu/PCu | 432 | −4.70 | 9 | −76 | 31 |
| R MTG/STG/PI/IPC | 1085 | −4.45 | 42 | −16 | −5 |
| L MTG/STG/PI | 520 | −4.44 | −60 | −28 | 13 |
| L/R SPL | 164 | −4.30 | 9 | −49 | 67 |
| R PCG | 137 | −4.05 | 48 | −10 | 52 |
| R/L mCG/SMA | 337 | −4.01 | 15 | −34 | 46 |
| R SFG | 105 | −3.93 | 24 | 14 | 55 |
Note: L: left; R: right. OC: occipital cortex; vmPFC: ventromedial prefrontal cortex; FPC: frontopolar cortex; Cu/PCu: cuneus/precuneus; MTG/STG/PI/IPC: middle temporal gyrus/superior temporal gyrus/posterior insula/inferior parietal cortex. SPL: superior parietal lobule; PCG: precentral gyrus; mCG/SMA: mid-cingulate gyrus/supplementary motor area; SFG: superior frontal gyrus. Negative Z values represent clusters with higher response to neutral vs. alcohol cues. Voxel P < 0.001, and cluster P<0.05 FWE corrected.
clusters with a voxel peak meeting P < 0.05, FWE-corrected and the cluster size represents number of voxels meeting this threshold.
Cue-elicited responses (alcohol vs. neutral) in relation to SCR
There was a total of 10 clusters identified from the one-sample t test of alcohol vs. neutral cue response (Table 2). In regions of interest (ROI) analyses, we extracted the β contrast for each cluster for a correlation with SCR (alcohol – neutral) and examined the results at a corrected p < 0.005 (0.05/10). The results of linear regressions showed that cue-elicited activation of the ventromedial prefrontal cortex (vmPFC; x=−6, y=44, z=−11) was significantly correlated with the difference in SCR between alcohol and neutral cue blocks (r=0.38, p=0.0022).
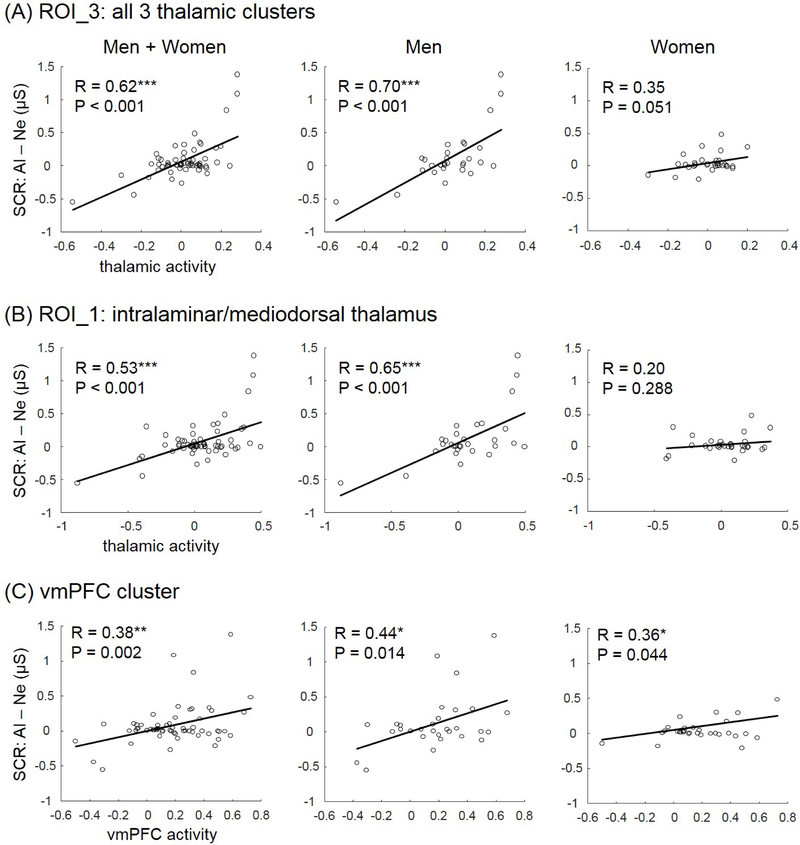
In addition to ROI analyses, we also conducted a whole-brain analysis with linear regression of the contrast (alcohol – neutral) against differences in SCR (alcohol – neutral, over the 36-s period) with age as a covariate. The results showed higher activities in a larger cluster encompassing subregions of the thalamus and the body of the caudate nucleus, as well as a small cluster in the cerebellum. Using a more stringent threshold of p<0.0001, uncorrected and cluster p<0.05 FWE-corrected, we identified three distinct clusters, including the left ventroposterior lateral thalamus (x=−21, y=−22, z=22, voxel Z=4.74, 26 voxels); right ventroposterior lateral thalamus (x=30, y=−28, z=7, voxel Z=4.66, 60 voxels); and intralaminar/mediosorsal thalamus (x=−6, y=−10, z=4, voxel Z=4.32, 37 voxels; shown in an inset of coronal sections, too) (Figure 2B). We extracted the beta contrast (alcohol – neutral) of a region of interest (ROI) combining all three clusters (ROI_3) and another ROI comprising solely the intralaminar/mediodorsal thalamus (ROI_1) for correlation and mediation analyses.
We examined the relationship between thalamic and vmPFC cue response and skin conductance response to alcohol vs. neutral cues for men and women separately to examine potential sex differences. The results showed that for both ROI_3 and ROI_1, thalamic cue response was significantly correlated with the SCR for men but not for women. A slope test confirmed the sex differences for ROI_3 (z=1.86, p=0.0314, one-tailed); and for ROI_1 (z=2.12, p=0.017, one-tailed). VmPFC cue response was positively correlated with SCR both in men (r=0.44, p=0.0140) and in women (r=0.36, p=0.0440). A slope test showed that the correlation did not differ significantly between men and women (z=0.35, p=0.7263). These results are shown in Figure 3.
Figure 3.
The correlation between SCR (alcohol – neutral) and thalamic cue response (alcohol – neutral) was significant for the entire sample, as expected. The correlation was also significant for men but not for women. (A) shows linear regressions based on ROI_3 of all three thalamic clusters (bilateral ventroposterior lateral and intralaminar/mediodorsal thalamsus) and (B) shows the regressions based on ROI_1 of the intralaminar/mediodorsal thalamus. (C) shows the regressions of the vmPFC cluster, as identified from region of interest analyses. Both thalamic clusters but not the vmPFC showed significant sex differences in the regression in slope tests (see text for statistics).
Cue-elicited responses (alcohol vs. neutral) in relation to craving rating
We also evaluated the neural correlates of inter-subject variation in cue-elicited craving in a whole-brain linear regression [i.e., with craving rating (alcohol – neutral cue) as the regressor]. At he same threshold of voxel p<0.001, uncorrected, the regression did not reveal any voxels in correlation with craving rating.
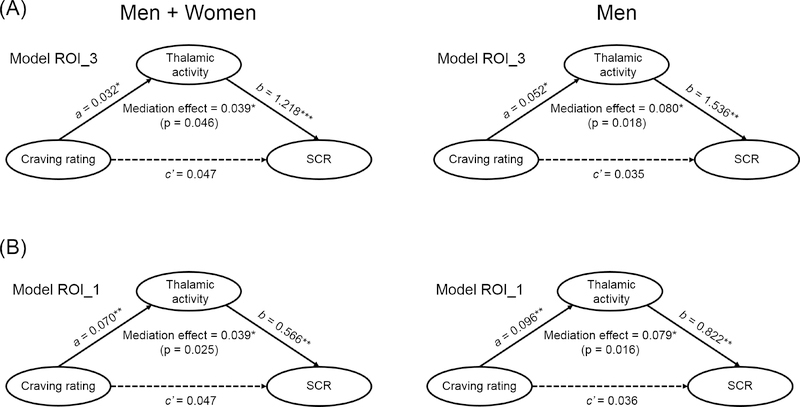
Mediation analysis
Subjective craving, thalamic/vmPFC cue response, and SCR were pair-wise positively correlated. Thus, we examined the inter-relationships using mediation analyses for men and women combined and for men alone. The findings of significant mediation are shown in Figure 4. For both ROI_3 and ROI_1, thalamic cue response mediated the correlation between cue-elicited craving and changes in SCR. None of the other five models showed significant mediation. For the vmPFC, no models showed significant mediation, although, as with the thalamus, the model craving -> neural activity -> SCR demonstrated the best p values, which were nearly significant (p=0.053 for men and women combined; p=0.112 for men). The results of all mediation analyses are shown in the Supplement.
Figure 4.
Mediation analyses showed that thalamic cue activity mediated the correlation between cue-evoked craving and skin conductance response (SCR) in men and women combined (left panels) and in men only (right panels). Thalamic cue activity was computed each of all of the three clusters (ROI_3, A) and the single cluster of the intralaminar/mediodorsal thalamus (ROI_1, B). Mediation analyses were not performed for women as the requirement that X was correlated both with M and Y was not met. The results of mediation for all other models were not significant (Supplementary Tables). The p values of mediation are shown in parentheses. Solid and dotted arrows each represent significant and non-significant correlations. * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
The current results showed that alcohol drinkers habituated to neutral but not to alcohol cues (Laberg et al., 1992) and, as a result, demonstrated higher skin conductance response (SCR) during alcohol vs. neutral cue exposure. These differences in cue evoked SCRs appeared to be carried largely by men, although a direct comparison failed to show significant sex differences. Further, the differences in SCR to alcohol vs. neutral cues were related to distinct regional responses in the thalamus, in men and women combined and in men but not women alone. Mediation analysis suggested that thalamic cue response mediated the relationship between cue induced craving and physiological reactivity. Together, these findings support a role of the thalamus in cue-elicited alcohol craving and increases in physiological arousal.
Thalamic responses to physiological arousal during cue exposure
Many cortical and subcortical regions respond to increases in physiological arousal during cognitive and emotional challenges and some of the regions respond specifically to task manipulations or psychological states. For instance, during biofeedback relaxation, anterior cingulate, amygdala, and insula activity was modulated by task manipulations that increased interoceptive processing, while variation in anterior insula activity reflected an interaction between accuracy and sensitivity of feedback (Critchley et al., 2002). In a study of the guilty knowledge test to examine the neural processes underlying deception, SCR amplitudes were linearly related to activity in the cerebellum, the right inferior frontal cortex, and the supplementary motor area (Gamer et al., 2007). A study of pain responses revealed brain activation more specifically associated with the SCR in the supracallosal ACC, amygdala, thalamus, and hypothalamus, distinct from regional activations to pain (Dube et al., 2009). Another study combined fMRI and measurements of electrodermal responses during an effortful motor task (MacIntosh et al., 2007). In addition to conventional hemodynamic modeling, skin conductance data have also used as time series to generate activation images from fMRI data. Whereas standard hemodynamic model demonstrated activities in the insula and cingulate cortices, modeling with skin conductance waveforms revealed additional activities in pre- and postcentral gyri, putamen and parietal cortices. Together, these studies suggest that the regional activities underlying SCR modulation may be task-dependent, likely because physiological arousal varies to task demands and are not readily separable from these manipulations and behaviors. In fact, some studies employed SCR to reflect participants’ awareness of task manipulations, which engaged different regional processes as compared to behavioral scenarios where participants were unaware of the manipulations (Klucken et al., 2009; Tabbert et al., 2010).
Here we showed that, exposure to alcohol as compared to neutral cues elicited regional responses that resemble those reported in earlier cue craving studies (Jasinska et al., 2014). For instance, activities in the visual and medial orbitofrontal cortices may reflect the saliency of alcohol vs. neutral cues. Although not showing increased response to alcohol vs. neutral cues, the thalamus showed higher activation in relation to differences in SCR to alcohol vs. neutral cues. Thus, the thalamus may play a specific role in associating cue exposure to increases in physiological arousal, broadly consistent with altered thalamic functions in drug and alcohol addiction (Huang et al., 2018). A closer examination showed a thalamic cluster concentrated in the intralaminar and mediodorsal nuclei, which are instrumental in regulating arousal and supporting a wide variety of cognitive functions (Hsu et al., 2014; Hsu and Price, 2007; Li and Kirouac, 2008; Van der Werf et al., 2002; Vogt et al., 2008).
The intralaminar thalamus receives dense noradrenergic projections from the locus coeruleus and projects to cortical regions, including the cingulate gyrus, and amygdala to support arousal and responses to salient events (Hsu et al., 2014; Hsu and Price, 2007; Li and Kirouac, 2008; Van der Werf et al., 2002; Vogt et al., 2008). Numerous studies demonstrated that electrical stimulation of the intralaminar thalamus or white matters connecting the intralaminar thalamus altered arousal and consciousness (Baker et al., 2016; Gummadavelli et al., 2015; Schiff, 2016). It is posited that, by integrating subcortical inputs and cortical responses, the intralaminar nucleus may facilitate synchronization of thalamic cortical activities, gating of attention, and higher level cognitive processes, including memory consolidation (Pereira de Vasconcelos and Cassel, 2015; Saalmann, 2014; Schiff et al., 2013; Varela, 2014). With its dense connections with prefrontal cortical strucutres, the mediodorsal nucleus is known to play a multifaceted role in higher cognitive functions (Mitchell, 2015; Ouhaz et al., 2018; Pergola et al., 2018). Alcohol cues elicits craving and cognitive and affective processing in relation to craving, and it remains to be investigated whether the extent of mediodorsal thalamic activation represents an urge to approach or a struggle to avoid alcohol. Together, it appears that, although a number of cortical and subcortical structures respond more strongly to alcohol as compared to neutral cues, the thalamus plays a unique role in supporting cue-evoked changes in physiological arousal.
Craving and physiological arousal
Cue reactivity has been a central topic in addiction research. Cue reactivity varies significantly across subjects, and the effect size of cue-evoked physiological responses was relatively small (Carter and Tiffany, 1999), as also shown in the current findings. In cocaine addicted individuals, physiological cue reactivity could not be related to a unitary state of high, withdrawal, or craving (Robbins et al., 1997). A study of methamphetamine-dependent subjects, drug cue-elicited craving was not correlated with physiological reactivity, as reflected by heart rate and skin conductance (Tolliver et al., 2010). Here, however, we demonstrated that cue-elicited craving and changes in SCR were correlated in men and women combined as well as in men but not in women alone. These findings suggest that the relationship between craving and physiological reactivity may potentially vary between substances and sexes. Indeed, a review of rodent studies showed that female rats, in general, acquire the self-administration of drugs and alcohol more rapidly, escalate drug taking more rapidly, show more motivational withdrawal and greater reinstatement, with the exception that female rats show less motivational withdrawal from (or “craving for”) alcohol (Becker and Koob, 2016).
The literature is limited on the neural processes linking craving and changes in arousal. An earlier study of cannabis users demonstrated higher cue-induced arousal, as indicated by significantly increased skin conductance and a larger late positivity of visual event-related brain potential (Wolfling et al., 2008). However, the physiological and neural reactivity were not correlated. To our knowledge, the current study was the first to query the whole brain for arousal-related regional activations to craving and highlight a specific role of the thalamus in linking craving and physiological manifestations in response to alcohol cues. The findings on the thalamus were broadly consistent with a recent rodent study demonstrating a critical role of thalamic projection to the dorsomedial striatum in the incubation of methamphetamine craving (Li et al., 2018). As discussed above, voxel cue activities peak at the intralaminar and mediodorsal nuclei, in accord with studies supporting these thalamic subregions in the regulation of arousal and other craving-related cognitive and affective processes (Hsu et al., 2014; Hsu and Price, 2007; Li and Kirouac, 2008; Van der Werf et al., 2002; Vogt et al., 2008).
Interestingly, a previous work showed that methylphenidate-induced craving for cocaine in cocaine-dependent subjects was correlated with reduced thalamic dopamine receptor availability (Volkow et al., 1997). A more recent study demonstrated that in alcohol-dependent individuals, citalopram as compared to saline resulted in decreased cue-induced craving for alcohol, and cue-induced alcohol craving was inversely correlated with thalamic but not striatal dopamine D2/3 receptor availability (Zorick et al., 2019). More studies are clearly warranted to understand the role of physiological arousal and its molecular bases in thalamic responses to craving.
It is worth noting that, although none of the mediation models were significant for the vmPFC, the model that showed the best p values were the same as with the thalamus, and these p values are nearly significant. It is possible that the small sample size may have limited the statistical power to identify a significant mediation.
Potential sex differences
Compared to men, women showed less craving as elicited by alcohol versus neutral cues. Although no sex differences were noted in the magnitude of SCR, men but not women demonstrated cue-elicited thalamic responses and a positive correlation between cue-elicited craving and SCR. These findings suggest that women may be less vulnerable to cue-induced alcohol craving and thalamic response to physiological arousal during craving, as compared to men, in broad consistency with a large body of literature as discussed earlier. Further, these findings appear to contrast with greater stress-related neural and physiological reactivity in women vs. men as demonstrated across many experimental conditions (Valentino et al., 2012), and suggest the importance of considering sex differences in studying the biological pathways underlying alcohol misuse (Barker and Taylor, 2017).
On the other hand, it is important to note that we did not record or control for menstrual cycling in female participants, who are known to demonstrate differences in electrodermal and neural responses during different menstrual phases (Goldstein et al., 2005; Gray et al., 2009; White and Graham, 2016). More broadly, men and women appeared to show differences in autonomic responses across a wide range of behavioral contexts (Finke et al., 2017; Hubbard et al., 2011; Williams et al., 2005). For instance, physiological reactivity in men but not women was influenced by whether the offers were framed as gain or loss in the Ultimatum Game (Sarlo et al., 2013). Men and women differed in facial electromyographic and autonomic reactions during self-relevance appraisal of gaze direction and dynamic facial expressions (Soussignan et al., 2013). Overall, the findings vary significantly across different experimental manipulations and physiological indices of autonomic responses and defy a simple account of sex differences in terms of higher or lower responses.
Limitations of the study and conclusions
A number of limitations need to be considered for the study. First, the study comprised a moderate sample size. Although whole-brain analyses in correlation with SCR demonstrated cue activations confined to the thalamus, we cannot rule out the possibility that a larger sample size may reveal other cortical and subcortical structures to support cue-elicited changes in physiological arousal. Likewise, the SCRs were highly variable, and the current results need to be confirmed in a larger sample. Second, as discussed earlier, we did not document menstrual cycle in female participants and it is possible that the lack of significant correlations between SCR and regional activities may reflect higher signal variability in women scanned during both follicular and luteal phases. Third, it remains unclear how cue-evoked changes in physiological arousal interact with other psychological constructs, such as impulsivity (Herman et al., 2018; Zhang et al., 2015), in determining alcohol use or how these cross-sectional findings may relate to future drinking behavior. Also, we did not document the time of last alcohol use, which may influence both cue-induced craving and imaging findings. Finally, the neural mechanisms underlying the potential sex differences are not clear. Studies have suggested roles of genetic underpinnings in neural responses to appetitive conditioning (Klucken et al., 2013) and of hormonal influences on motivation-related addictive behavior (Becker and Koob, 2016). New experiments and longitudinal follow-ups are required to address these questions.
To conclude, we showed that thalamic cue activity was positively correlated with differences in SCR between alcohol and neutral cue blocks and mediated the correlation between craving and SCR. These findings appear to be carried primarily by male drinkers, who demonstrated higher craving response to alcohol cues than female drinkers. Together, the findings substantiate physiological and neural correlates of alcohol cue response and suggest important sex differences in the physiological and neural processes of cue evoked alcohol craving.
Supplementary Material
Acknowledgements and disclosures
The study was supported by NIH grants AA021449 and P50AA12870, and the VA National Center for PTSD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. JK has individual consultant agreements at less than $10,000 per year with AstraZeneca Pharmaceuticals, Biogen, Idec, MA, Biomedisyn Corporation, Bionomics, Limited (Australia), Boehringer Ingelheim International, Concert Pharmaceuticals, Inc., Heptares Therapeutics, Limited (UK), Janssen Research & Development, L.E.K. Consulting; Otsuka America Pharmaceutical, Inc., Spring Care, Inc., Sunovion Pharmaceuticals, Inc., Takeda Industries, andTaisho Pharmaceutical Co., Ltd. JK is on the scientific advisory board of Bioasis Technologies, Inc., Biohaven Pharmaceuticals; Blackthorn Therapeutics, Inc., Broad Institute of MIT and Harvard, Cadent Therapeutics, Lohocla Research Corporation, Pfizer Pharmaceuticals, and Stanley Center for Psychiatric Research at the Broad Institute. JK has stocks or stock options on ArRETT Neuroscience, Inc., Blackthorn Therapeutics, Inc., Biohaven Pharmaceuticals Medical Sciences, Spring Care, Inc., Biohaven Pharmaceuticals Medical Sciences. JK receives income greater than $10,000 as the editor of Biological Psychiatry. JK has the following patents or patent applications: Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia (US Patent #:5,447,948); Glutamate Modulating Agents in the Treatment of Mental Disorders (US Patent No. 8,778,979); Intranasal Administration of Ketamine to Treat Depression (No. 14/197,767 filed on March 5, 2014); Methods for Treating Suicidal Ideation (No. 14/197.767 filed on March 5, 2014); Composition and methods to treat addiction (No.61/973/961. April 2, 2014); Treatment Selection for Major Depressive Disorder (filed June 3, 2016, USPTO docket number Y0087.70116US00); Compounds, Compositions and Methods for Treating or Preventing Depression and Other Diseases (No. 62/444,552, filed on January10, 2017); Combination Therapy for Treating or Preventing Depression or Other Mood Diseases (No. 047162–7177P1 (00754) filed on August 20, 2018). JK receives nonfederal research support: AstraZeneca Pharmaceuticals provides the drug, Saracatinib, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism; Pfizer Pharmaceuticals provides an investigational drug, PF-03463275, for research related to NIH grant “Translational Neuroscience Optimization of GlyT1 Inhibitor.” The other authors do not have financial interests to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, 2001. The alcohol use disorders identification test. World Health Organization, Department of Mental Health and Substance Dependence, Geneva. [Google Scholar]
- Baker JL, Ryou J-W, Wei XF, Butson CR, Schiff ND, Purpura KP, 2016. Robust modulation of arousal regulation, performance, and frontostriatal activity through central thalamic deep brain stimulation in healthy nonhuman primates. Journal of neurophysiology 116(5), 2383–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, 2017. Sex differences in incentive motivation and the relationship to the development and maintenance of alcohol use disorders. Physiol Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68(2), 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Ecker AH, Jeffries ER, 2016. Cannabis craving in response to laboratory-induced social stress among racially diverse cannabis users: The impact of social anxiety disorder. J Psychopharmacol 30(4), 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, Cinciripini PM, 2009. Generalized craving, self-report of arousal, and cue reactivity after brief abstinence. Nicotine & Tobacco Research 11(7), 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST, 1999. Meta-analysis of cue-reactivity in addiction research. Addiction 94(3), 327–340. [PubMed] [Google Scholar]
- Ceylan-Isik AF, McBride SM, Ren J, 2010. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life sciences 87(5–6), 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan A, O’Brien C, 1988. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr 81, 74–80. [PubMed] [Google Scholar]
- Critchley HD, 2002. Electrodermal responses: What happens in the brain. Neuroscientist 8(2), 132–142. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ, 2002. Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage 16(4), 909–919. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nagai Y, Gray MA, Mathias CJ, 2011. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton Neurosci 161(1–2), 34–42. [DOI] [PubMed] [Google Scholar]
- Dube A-A, Duquette M, Roy M, Lepore F, Duncan G, Rainville P, 2009. Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage 45(1), 169–180. [DOI] [PubMed] [Google Scholar]
- Figner B, Murphy RO, 2011. Using skin conductance in judgment and decision making research. A handbook of process tracing methods for decision research, 163–184. [Google Scholar]
- Finke JB, Deuter CE, Hengesch X, Schachinger H, 2017. The time course of pupil dilation evoked by visual sexual stimuli: Exploring the underlying ANS mechanisms. Psychophysiology 54(10), 1444–1458. [DOI] [PubMed] [Google Scholar]
- First MS,R; Williams J; Gibbon M, 1995. Structured Clinical Interview for DSM-IV (SCID). American Psychiatric Association, Washington DC. [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R, 2012. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol 26(7), 958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Bauermann T, Stoeter P, Vossel G, 2007. Covariations among fMRI, skin conductance, and behavioral data during processing of concealed information. Human Brain Mapping 28(12), 1287–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WH, Frone MR, Cooper ML, Russell M, Skinner JB, Windle M, 1995. A revised Alcohol Expectancy Questionnaire: factor structure confirmation, and invariance in a general population sample. Journal of studies on alcohol 56(2), 177–185. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N, 2005. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience 25(40), 9309–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe SD, Upadhyaya HP, 2009. Menstrual cycle and cue reactivity in women smokers. Nicotine & Tobacco Research 12(2), 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummadavelli A, Motelow JE, Smith N, Zhan Q, Schiff ND, Blumenfeld H, 2015. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia 56(1), 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Herman AM, Critchley HD, Duka T, 2018. The role of emotions and physiological arousal in modulating impulsive behaviour. Biol Psychol 133, 30–43. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta J-K, Bhatnagar S, 2014. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Frontiers in behavioral neuroscience 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL, 2007. Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology 504(2), 89–111. [DOI] [PubMed] [Google Scholar]
- Huang AS, Mitchell JA, Haber SN, Alia-Klein N, Goldstein RZ, 2018. The thalamus in drug addiction: from rodents to humans. Phil. Trans. R. Soc. B 373(1742), 20170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CS, Ornitz E, Gaspar JX, Smith S, Amin J, Labus JS, Kilpatrick LA, Rhudy JL, Mayer EA, Naliboff BD, 2011. Modulation of nociceptive and acoustic startle responses to an unpredictable threat in men and women. Pain 152(7), 1632–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y, 2014. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaag AM, Wiers RW, de Vries TJ, Pattij T, Goudriaan AE, 2018. Striatal alcohol cue-reactivity is stronger in male than female problem drinkers. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Kagerer S, Schweckendiek J, Tabbert K, Vaitl D, Stark R, 2009. Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture–picture conditioning paradigm. Neuroscience 158(2), 721–731. [DOI] [PubMed] [Google Scholar]
- Klucken T, Wehrum S, Schweckendiek J, Merz CJ, Hennig J, Vaitl D, Stark R, 2013. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Hum Brain Mapp 34(10), 2549–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberg JC, Hugdahl K, Stormark KM, Nordby H, Aas H, 1992. Effects of visual alcohol cues on alcoholics’ autonomic arousal. Psychol Addict Behav 6(3), 181. [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, Ma MY, Xue MM, Luo YX, Yang FD, 2015. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol 20(3), 513–522. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ, 2008. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. Journal of Comparative Neurology 506(2), 263–287. [DOI] [PubMed] [Google Scholar]
- Li X, Witonsky KR, Lofaro OM, Surjono F, Zhang J, Bossert JM, Shaham Y, 2018. Role of Anterior Intralaminar Nuclei of Thalamus Projections to Dorsomedial Striatum in Incubation of Methamphetamine Craving. J Neurosci 38(9), 2270–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh BJ, Mraz R, McIlroy WE, Graham SJ, 2007. Brain activity during a motor learning task: An fMRI and skin conductance study. Human brain mapping 28(12), 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ, 2004. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage 22(1), 243–251. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A, 2006. Skin conductance: A psychophysiological approach to the study of decision making. Methods in mind, 103–122. [Google Scholar]
- Pachas GN, Gilman J, Orr SP, Hoeppner B, Carlini SV, Loebl T, Nino J, Pitman RK, Evins AE, 2015. Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology 232(9), 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, Cassel JC, 2015. The nonspecific thalamus: A place in a wedding bed for making memories last? Neurosci Biobehav Rev 54, 175–196. [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Verbanck P, Campanella S, 2013. Gender differences in reactivity to alcohol cues in binge drinkers: A preliminary assessment of event-related potentials. Psychiat Res 209(3), 494–503. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, Cornish JW, O’Brien CP, 2000. Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug Alcohol Depen 59(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP, 1997. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav 22(2), 157–167. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD, 1994. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol 55(4), 487–494. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, 2014. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlo M, Lotto L, Palomba D, Scozzari S, Rumiati R, 2013. Framing the ultimatum game: gender differences and autonomic responses. Int J Psychol 48(3), 263–271. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18(1), 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, 2016. Central thalamic deep brain stimulation to support anterior forebrain mesocircuit function in the severely injured brain. Journal of Neural Transmission 123(7), 797–806. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Shah SA, Hudson AE, Nauvel T, Kalik SF, Purpura KP, 2013. Gating of attentional effort through the central thalamus. J Neurophysiol 109(4), 1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA, 2008. From fear to safety and back: reversal of fear in the human brain. J Neurosci 28(45), 11517–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R, 2011. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp 32(11), 1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM, 2009. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34(5), 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussignan R, Chadwick M, Philip L, Conty L, Dezecache G, Grezes J, 2013. Self-relevance appraisal of gaze direction and dynamic facial expressions: effects on facial electromyographic and autonomic reactions. Emotion 13(2), 330–337. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Andrade KC, Schroter MS, Sturm A, Goya-Maldonado R, Samann PG, Czisch M, 2011. The neural correlates of negative prediction error signaling in human fear conditioning. Neuroimage 54(3), 2250–2256. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, Stark R, 2010. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Soc Cogn Affect Neur 6(4), 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, Baker NL, Brady KT, 2010. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am J Drug Alcohol Abuse 36(2), 106–113. [DOI] [PubMed] [Google Scholar]
- Trotzke P, Starcke K, Pedersen A, Brand M, 2014. Cue-induced craving in pathological buying: empirical evidence and clinical implications. Psychosomatic medicine 76(9), 694–700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D, 2012. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology 62(1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ, 2002. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39(2–3), 107–140. [DOI] [PubMed] [Google Scholar]
- Varela C, 2014. Thalamic neuromodulation and its implications for executive networks. Front Neural Circuits 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Friedman DP, Sikes RW, Vogt LJ, 2008. Norepinephrinergic afferents and cytology of the macaque monkey midline, mediodorsal, and intralaminar thalamic nuclei. Brain Structure and Function 212(6), 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EC, Graham BM, 2016. Estradiol levels in women predict skin conductance response but not valence and expectancy ratings in conditioned fear extinction. Neurobiology of learning and memory 134, 339–348. [DOI] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, Bryant RA, 2005. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 28(3), 618–626. [DOI] [PubMed] [Google Scholar]
- Willner P, Field M, Pitts K, Reeve G, 1998. Mood, cue and gender influences on motivation, craving and liking for alcohol in recreational drinkers. Behav Pharmacol 9(7), 631–642. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM, 2008. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci 27(4), 976–983. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, Li C.-s.R., 2013. Ventromedial prefrontal cortex and the regulation of physiological arousal. Soc Cogn Affect Neur 9(7), 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Hu J, Wu PL, Chao HH, Li CS, 2015. Barratt Impulsivity and Neural Regulation of Physiological Arousal. Plos One 10(6), e0129139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Fan C, Du J, Jiang H, Chen H, Sun H, 2012. Cue-induced craving and physiological reactions in recently and long-abstinent heroin-dependent patients. Addict Behav 37(4), 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le T, Leeman RF, Bi J, Krystal JH, Li C.-s.R., 2018. Alcohol expectancy and cerebral responses to cue-elicited craving in adult non-dependent drinkers. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.