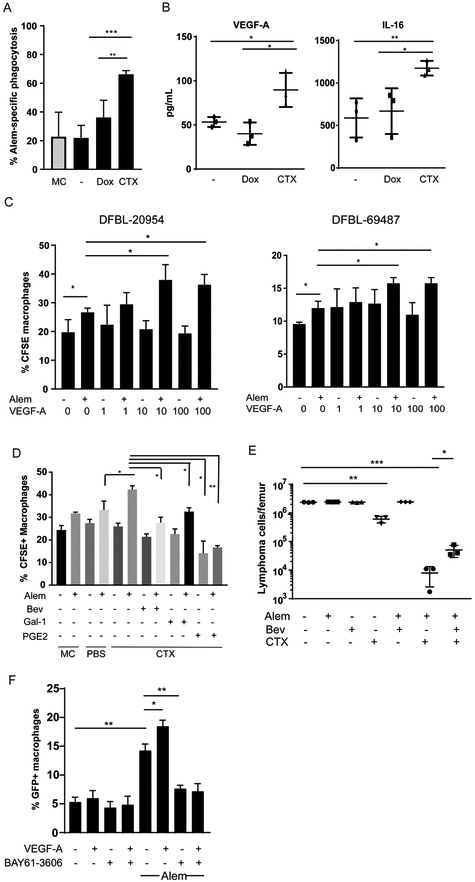
Figure 4: Cyclophosphamide Induces DHL Secretion of VEGF-A and IL-16 to promote tumor clearance.
(A) DFBL-20954 cells were isolated from BM of untreated mice, labeled with CFSE and co-cultured with bone-marrow derived macrophages and conditioned media from mice (n=3 per condition) treated as indicated. MC = media control. Alem specific killing is defined as the % increase in CFSE+ macrophages in the presence of Alem compared to no antibody. Unpaired two-sided-t-test, **p<0.01, ***p<0.001
(B) Levels of human VEGF-A and IL-16 from bone marrow of DFBL-20954-engrafted mice collected 16 hours after in vivo treatment with vehicle (−), doxorubicin (Dox) or cyclophosphamide (CTX). Representative experiment shown. Levels of murine VEGF-A by the same cytokine arrays were <1 pg/ml. Unpaired two-sided-t-test, *p<0.05, **p<0.01.
(C) BMDMs were incubated with CFSE-labeled BM lymphoma cells (N=3 mice) from untreated mice supplemented with Alem, recombinant human VEGF-A or vehicle (−). Experiments were performed ≥2 times and representative examples are shown. Phagocytosis was assessed as the percent of CD11b+ cells that were CFSE+. Two-sided Welch’s t-test, *p<0.05.
(D) BMDMs were incubated with CFSE-labeled BM lymphoma cells from untreated mice and conditioned media (n=3 mice per condition) supplemented with vehicle (−), Alem, recombinant Gal-1 (1 ¼g/ml), PGE2 (5 ng/ml), and/or bevacizumab (bev, 30 ¼g/ml). Unpaired two-sided-t-test.
(E) Bone marrow tumor burden of DFBL-20954-engrafted mice on day 8 after treatment with the indicated agents. Two-sided Welch t-test.
(F) BMDMs were incubated with GFP+ Raji cells and recombinant human VEGF-A (10ng/ml) in the presence and absence of Alem. The experiment was performed three times and a representative example is shown. The SYK inhibitor BAY61-3606 (20 nM) was added as indicated. Phagocytosis was assessed as the percent of CD11b+ cells that were GFP+. Unpaired two-sided-t-test.