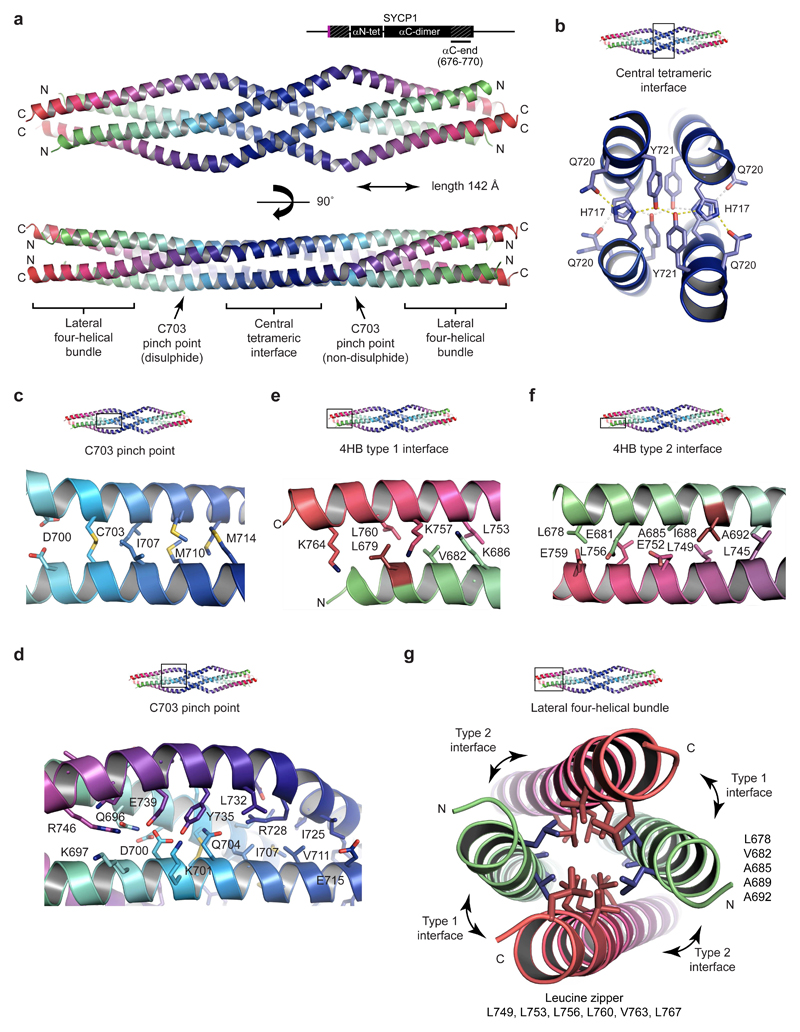
Figure 5. Crystal structure of the SYCP1 C-terminal tetrameric assembly.
(a) Crystal structure of SYCP1 αC-end (676-770) in crystal form 1, demonstrating an anti-parallel tetrameric assembly of length 142 Å. The structure includes a central tetrameric interface flanked by C703 pinch points that lead to lateral four-helical bundles. N- and C-termini are coloured in green and red respectively. (b) The central tetrameric interface consists of two stacked layers each containing a hydrogen bonding network of pairs of H717, Q720 and Y721 residues. (c-d) The C703 pinch point consists of a parallel dimeric coiled-coil (containing C703) flanked by surrounding anti-parallel chains. (c) The parallel dimeric coiled-coil is formed of heptad residues D700, C703, I707, M710 and M714 (d) The flanking chains have a distinct angulation at E731 and provide pseudo-cores of loose anti-parallel interactions. (e-g) The lateral four-helical bundle (4HB) is formed of a hydrophobic core and anti-parallel interfaces. (e) The lateral 4HB type 1 interface is an anti-parallel coiled-coil of heptad residues L679, V682 and K686, L753, K757, L760 and K764. (f) The lateral 4HB type 2 interface is an anti-parallel coiled-coil of heptad residues L678, E681, A685, I688 and A692, L745, L749, E752, L756 and E759. (g) Cross-section through the lateral 4HB assembly. A hydrophobic core is formed from residues that also contribute to 4HB anti-parallel interfaces and are predicted to mediate the formation of N- and C-terminal parallel dimeric coiled-coils in the non-assembled conformation. L679 and I688 are the only hydrophobic 4HB residues not also implicated in the putative parallel dimeric coiled-coil structure.