Abstract
It has been suggested that giant Antarctic marine invertebrates will be particularly vulnerable to declining O2 levels as our ocean warms in line with current climate change predictions. Our study provides some support for this oxygen limitation hypothesis, with larger body sizes being generally more sensitive to O2 reductions than smaller body sizes. However, it also suggests that the overall picture is a little more complex. We tested predictions from three different, but overlapping, O2-related hypotheses accounting for gigantism, using four Antarctic amphipod species encompassing a wide range of body sizes. We found a significant effect of body size, but also of species, in their respiratory responses to acutely declining O2 tensions. The more active lifestyle of intermediate-sized Prostebbingia brevicornis was supported by a better respiratory performance than predicted by the oxygen limitation hypothesis alone, but consistent with the symmorphosis hypothesis. We suggest that giant polar amphipods are likely to be some of the first to fare badly in an O2-poor ocean. However, the products of past evolutionary innovation, such as respiratory pigments that enhance O2-transport and novel gas exchange structures, may in some species offset any respiratory disadvantages of either large or small body size.
This article is part of the theme issue ‘Physiological diversity, biodiversity patterns and global climate change: testing key hypotheses involving temperature and oxygen’.
Keywords: oxygen limitation hypothesis, Symmorphosis, respiratory advantage hypothesis, oxyregulation, gigantism, global climate change
1. Introduction
It is widely recognized that climate change will impact marine biodiversity in manifold and complex ways, and there will be winners and losers [1–3]. Understanding the biological mechanisms determining such outcomes is paramount, especially if those mechanisms generalize across taxa [4–6]. In particular, determining the physiological features that might be associated with gigantism and how giant aquatic animals will fare in a future oxygen (O2)-poor ocean [7] have attracted attention [8–14]. One longstanding notion is that living in cold, O2-rich waters may favour the evolution of more larger-bodied species, compared with living in warmer (and so containing less dissolved O2) temperate and tropical waters. De Broyer [15] showed that about one-third of Antarctic amphipods had body sizes twice that of the mean body size in their genus. However, it was Chapelle & Peck [8] who first established, and subsequently strengthened [16,17], the evidence for a quantitative relationship between maximum body size and environmental O2. They championed the idea that polar gigantism is possible because (1) more O2 is available in cold, compared with warm, waters and (2) there is reduced metabolism (and so low rates of O2 uptake [V̇O2]) at such sub-zero temperatures. Based on the strength of these relationships, they formulated their ‘oxygen (limitation) hypothesis', i.e. maximum potential body size is limited by O2 availability. They went on to suggest that, ‘(g)iant amphipods may therefore be among the first species to disappear if global temperatures are increased or global O2 levels decline’. Since then their oxygen limitation hypothesis has attracted attention, including empirical tests of its key prediction that, all else being equal, reduced O2 concentration should have a disproportionately large effect on the performance of large-bodied species. While tests of this prediction have provided some support (e.g. [9,18–20]), the evidence remains equivocal, with seemingly different outcomes for animals that do or do not possess specialized respiratory gas exchange surfaces and transport mechanisms [12,21–23] as not always is ‘all else equal’ [9].
Since then, two alternative hypotheses linking O2 to body size have been suggested. The first is that animals of different body size should show symmorphosis (sensu Weibel et al. [24]), i.e. their respiratory systems have been the subject of evolutionary change and, irrespective of body size, have been shaped to supply adequate O2 for metabolism, minimizing redundant excess capacity [9]. The ‘symmorphosis hypothesis' predicts that any effects of reduced O2 should be independent of body size. If supported, this would be the death-knell of the idea of relating size to vulnerability [25]. The second is the ‘respiratory advantage hypothesis’ [10]. This is predicated on the seemingly counterintuitive idea that despite reduced metabolic demand and greater O2 solubility in cold waters, O2 bioavailability is lower. This is because any potential benefit of increased O2 solubility in the cold may be negated by a concomitant reduction in the O2 diffusion coefficient, the coefficient affected by a combination of the reduction in the thermal motion of O2 molecules and increased viscosity at low temperatures [26]. Therefore, large body size in an animal that does not have well-developed mechanisms for respiratory regulation (e.g. possession of a respiratory pigment like haemoglobin/haemocyanin or well-developed hyperventilation response) bestows a respiratory advantage that can overcome viscosity-related, low O2 availability in polar waters [10]. Consequently, one prediction from the respiratory advantage hypothesis is that gigantism should be absent in polar groups characterized by good respiratory control. A second prediction is that reduced O2 should have a disproportionately large effect on the performance of small-bodied individuals.
Moran & Woods [25] identify at least eight hypotheses explaining ‘gigantism’. While it is unlikely that body size variation generally, and gigantism specifically, can be attributable to simple or univariant explanations [25,27], the three O2 theories outlined above are testable, and amphipod crustaceans in particular, given their relatively conservative body plan and the fact they have already contributed much to our understanding, make excellent models. Therefore, the aim of this present study was to test the predictions arising from these three hypotheses using carefully selected amphipod species. The main performance measure chosen was the ability to maintain respiratory independence during exposure to acutely declining PO2s. It does not seem unreasonable to assume that such respiratory independence is a good fitness proxy given the importance of aerobic metabolism in a relatively active group such as the amphipods.
All other measures are features that underpin that respiratory performance, with a view to determining how much ‘all else is equal’. (1) The ventilation response to acutely declining PO2s was quantified as this underpins the respiratory performance measure, at least acutely although in more hypoxic environments it may also be a chronic response [28]. (2) Total gill area was quantified for three of the species as it is possible that larger species, if their physiology is compromised by internal hypoxia, could have developed a greater mass-specific gill area. Prostebbingia brevicornis, in common with some other amphipod species (e.g. [29,30]), appeared to have extrabranchial gas exchange surfaces (EGS) in the form of modified coxal plates. This was the only one of the four species that displayed this modification. Therefore, their coxal gas exchange area was estimated and expressed as a proportion of total gill area. (3) Finally, evidence was sought for the presence of a respiratory pigment that would improve O2 transport from the gills to the tissues, and potentially contribute to respiratory performance during hypoxia.
Studies of the activity of Antarctic marine ectotherms, to date, show that activity rates are generally low, with a few notable exceptions where adaptations, such as increased mitochondrial densities, have permitted locomotory speeds to be maintained [31]. In the majority of species, where the activity is limited by the constant cold of the Southern Ocean, symmorphosis predicts that their respiratory organs will have lost the capacity to respond to the increased O2 demand caused by elevated metabolic rates at warmer body temperatures [32]. Tests of the thermal response of locomotion can therefore be used to assess the temperature sensitivity of activity, which in turn may provide insights into the flexibility of O2 supply [19].
Four species of widely differing body sizes were chosen (species name and recorded wet mass range from the field): Paraceradocus miersi (0.042–2.7 g), Shraderia gracilis (0.038–0.062 g) Probulisca ovata (0.003–0.001 g) and Prostebbingia brevicornis (0.037–0.103 g) (see electronic supplementary material, S1 for further details). The first three were selected because they are phylogenetically distant from one another (three different superfamilies); all of them appear similarly sluggish at low temperatures, but they encompass a very wide range of body sizes. Access to congeneric species covering a wider range of body sizes, or investigating a large enough number of species of known phylogeny to incorporate phylogenetic non-independence, would have been ideal but neither option proved possible. The fourth species, P. brevicornis, was chosen because it has a larger body size than the relatively closely related, S. gracilis (same family) and its size range overlaps with two of the other species. It also appears to be considerably more active and has a different body form from the other three species.
These four species are abundant and co-occur in the same habitat, a relatively shallow-water (6–8 m depth) boulder field close to Rothera Research Station, in coastal waters of the western Antarctic Peninsula (electronic supplementary material, S1). There is also some evidence that even when overlying water is normoxic, small individuals living beneath those boulders may experience reduced PO2s (electronic supplementary material, S1).
2. Materials and methods
(a). Animal material
Amphipods were collected close to the British Antarctic Survey research station, Rothera Point, on the western Antarctic Peninsula (see electronic supplementary material, S1 for collection details). Upon capture, amphipods were transferred to containers filled with seawater at the same temperature as their surroundings (T = 0°C) and taken to the laboratory within 60 min of capture. Here amphipods were maintained in a number of Aquarium Tank Fish Hatchery and Fry Breeder Units (All Pond Solutions, Uxbridge, UK) that floated, partially submerged, in large aquaria supplied with untreated running seawater (T = −1°C to 0°C, S = 35, DO = 100–106% air saturation) pumped directly from South Cove adjacent to the laboratory. Each unit was supplied with squares of plastic mesh that provided shelter and greatly reduced cannibalism and agonistic interactions. All amphipods were kept unfed for at least 48 h under these conditions before being used in any of the experiments described below.
(b). Measurement of V̇O2 during acutely declining PO2s
Rates of O2 uptake during acutely declining PO2s were measured (T = −1.0°C for all four species using a well-established, closed respirometer technique [33]; also see electronic supplementary material, S2A). This technique was compared with another well-established but semi-open technique that has been used extensively for amphipods [34]. Two of the Antarctic species examined in this current study (P. miersi and P. brevicornis) were collected during the previous season and returned live from Antarctica to Plymouth where the comparison took place. There was no significant difference in V̇O2s between the techniques (electronic supplementary material, S2A).
Immediately the respirometer bottles were sealed, each containing an individual amphipod, the water was gently stirred using a magnetic stirrer (Mighty mouse IKAR colour squid) and the O2 concentration in the water measured. Oxygen concentration (sensitivity 0.1%) was measured using a Presens system (Fibox 4 Precision Sensing GmbH, Regensburg, Germany). Concurrently, ventilation rate was quantified visually using a hand-held magnifying glass (×10) and stopwatch. The time taken for 20 beats was measured in triplicate and an average calculated. Oxygen and ventilation measurements were made at 30 min intervals until all of the O2 was exhausted within the bottle or until there were no visible signs of movement from the amphipod (movement of pleopods, pereopods or antennae). At this time, individuals were removed, carefully blotted dry and weighed using a precision semi-microbalance (Genius ME235S, Sartorius, Bradford, MA). V̇O2 was calculated as μL g wet mass–1 h–1, but in species comparisons of the effect of acutely declining PO2s, uptake was expressed as the percentage of the mean normoxic rate for that species, at the corresponding environmental PO2 (expressed as % air saturation).
(c). Quantifying oxyregulatory ability
To characterize the relationship between V̇O2 and environmental PO2 for individuals of each species, we calculated a regulation index (RI), a novel measure of oxyregulation proposed by Mueller & Seymour [35]. Several techniques have been suggested for comparing oxyregulatory ability in aquatic animals (e.g. [36,37]) but with no general agreement on which is best. The RI was chosen because the measure is quantitative and more appropriate for working with species that show relatively poor oxyregulatory ability. Briefly, V̇O2 (as % normoxic rate) was plotted as a function of environmental PO2 for each individual, and in each case, a line was fitted using a third-order polynomial that best described the curve. The area enclosed by this line of best fit and a straight line describing the relationship that would be present if the individual showed absolute oxyconformity were quantified using integration (using Graph Pad, Prism 7). An RI = 1 represents perfect oxyregulation, 0 perfect oxyconforming (see electronic supplementary material, S2 for examples). Thus, calculated values within this range are good measures of respiratory independence under conditions of acutely declining PO2s. A mean RI value was calculated for each individual from each species. We then tested for an effect of body mass (as a fixed factor) on RI (as response variable) controlling for species (as a random factor).
(d). Measurement of gill and extrabranchial exchange surface areas
Gill area measurements were carried out on fresh material of P. miersi (n = 6) P. brevicornis (n = 9) and S. gracilis (n = 9) (but not successfully on P. ovata as they were so small) following closely the method of Moore & Taylor [38]. This involved carefully excising each gill from individual amphipods, encompassing a wide range of body masses (0.016–3.30 g wet mass). Gills were carefully removed from the base of the walking legs using fine forceps. Excised gills were mounted in seawater (S = 35) on a microscope slide without a coverslip, and photographed under low-power magnification (×10–40) (Nikon D7000; Niko SM2800) using a camera attachment and cold light source (Schott, KL1500). Each digital image was calibrated using a stage micrometer (100 × 0.1 = 10 mm, Graticules Ltd, Tonbridge, Kent, England) and areas were calculated using Image J.
Total gill area was expressed as the sum of the individual areas (×2) and regressed after double logarithmic transformation on body dry mass using the method of least squares.
Dry body mass was estimated by interpolation from a regression equation fitted to log wet mass/log dry mass data (electronic supplementary material, S3).
(e). Extrabranchial gas exchange surfaces
The same technique used to measure the gill area was also used to estimate surface areas of excised coxal plates of P. brevicornis (n = 5). Based on the fact that these plates were so thin, and there is evidence of their being used in gas exchange in other amphipods, they were considered putative EGS (electronic supplementary material, S4).
(f). Evidence for respiratory pigment in the haemolymph
Haemolymph was obtained by direct cardiac puncture from individuals of all species, except P. ovata. Even the largest individual of P. ovata was too small to sample for haemolymph. Haemolymph was collected using a microsyringe (Hamilton, vol. = 10 or 25 µl), the needle of which was inserted dorsally between the three and four pereon segments. Samples were transferred to a microcentrifuge tube (Eppendorf vol. = 0.5 ml) on ice. While individual haemolymph samples were obtained for P. miersi (15–60 µl), because of the small volumes obtained from Prostebbingia and Shraderia (0.5–6 µl per individual), it was necessary to pool samples. Haemolymph samples were then diluted 1 : 100 and their absorbance over the range λ = 260–600 nm determined using the scan function of a UV–VIS spectrophotometer (Helios Gamma, Thermo spectronic, Cambridge). Both samples and blanks were tested in precision microcuvettes (Quartz SUPRASIL Hellma, 1 cm pathlength).
(g). Rates of crawling and swimming
The activity rates of 10 individuals of the three largest species, P. miersi, P. brevicornis and S. gracilis were recorded at different test temperatures (nominally 0.0, 3.0, 6.0 and 9.0°C) to investigate relationships between the thermal response of both crawling and bursts of rapid swimming with measures of respiratory performance. Initial trials showed that only P. brevicornis moved using both burst swimming and crawling. P. ovata was too small to reliably track its activity from the video recordings.
Swimming and crawling speeds were measured as follows. A 70 mm tall plastic ring (height = 70 mm tall, circumference = 0.82 m) was placed into seawater of 40 mm depth. This ring was placed on a 50 × 50 mm grid so that swimming and crawling speed could be calculated from video recordings. Recordings were made using a LifeCam Cinema HD video camera (Microsoft) at 25 frames s–1 using VideoVelocity 3 software (Candy Labs). This software stamped a time code onto the video recording that allowed swimming speed to be measured by manually plotting the distance moved between frames of known time intervals, where distance was calibrated from the closest grid line to the amphipod. Up to 10 swimming or crawling sequences were captured and analysed for each individual. Individual body lengths were measured and swimming and crawling speeds expressed as units of body lengths per second.
Seawater temperature was maintained within a jacketed aquarium (as described previously) and the temperature raised from ambient (i.e. 0.0 ± 0.2°C, mean ± 1 s.d.) at 0.1°C h–1. Once the test temperature was achieved, it was held for 24 h, allowing the physiology of individuals to stabilize to the test temperature before swimming trials were conducted.
3. Results
(a). V̇O2 and ventilation during hypoxia
Patterns of V̇O2 and ventilation during acutely declining PO2s, for each of the four amphipod species, are presented in figure 1 and the calculated RI for each species in figure 2. There was a significant effect of body size on RI (F1,35 = 4.84, p = 0.035) but also a significant effect of species identity (F3,75 = 41.57, p < 0.001). In summary, respiratory performance under hypoxia decreased with increasing body size, with P. brevicornis as an exception.
Figure 1.
Rates of O2 uptake and ventilatory activity of four Antarctic species exposed to acutely declining environmental PO2. Values and means ±1 s.d. (n = 6 in each case). Dashed lines = lines of oxyconformity.
Figure 2.
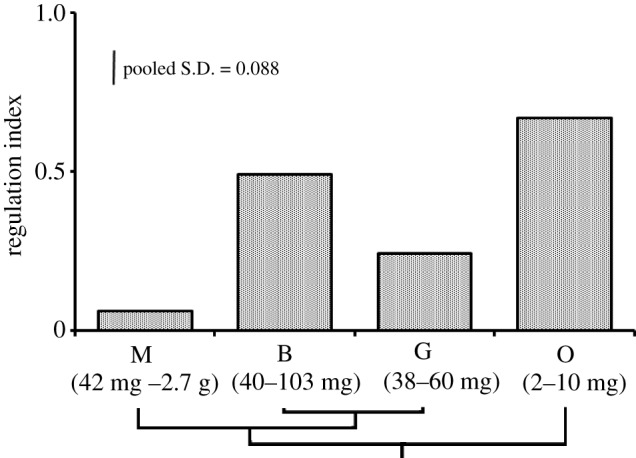
Regulation index (RI) for each of the four Antarctic amphipod species studied, indicating the phylogenetic relatedness of the species, and the body size categories (wet mass) of all individuals examined. All data are normally distributed (Anderson–Darling ≤ 0.297, p ≥ 0.347) and variances were not significantly different (multiple comparisons, p = 0.347). M = P. miersi, B = P. brevicornis, G = S. gracilis, O = P. ovata.
The species with the largest body size, P. miersi showed no oxyregulation and there was possibly evidence of hypometabolism in some of the largest individuals. P. miersi therefore had the lowest calculated RI, and while RI appeared to increase with decreasing body size within the species, when tested this was not significant (F1,11 = 0.25, p = 0.626). It mounted a poor hyperventilatory response (max. 12% increase at 60–55% air saturation (a.s.)), which remained almost unchanged, even when in anoxia. The smallest species, P. ovata, possessed the strongest pattern of oxyregulation and so the highest calculated RI (figure 2). Unfortunately, it was not possible to quantify its hyperventilatory response as the pleopods were not fully visible under experimental conditions.
Both P. brevicornis and S. gracilis were characterized by body sizes smaller than the largest P. miersi but larger than P. ovata and displayed oxyregulatory patterns intermediate to the smallest and largest species examined. Unlike P. miersi, both P. brevicornis and S. gracilis showed a pronounced hypoxia-related hyperventilation. However, the larger species, P. brevicornis, had a significantly greater calculated RI (controlling for body mass) and showed a slightly better developed hyperventilatory response than the smaller species S. gracilis. These patterns of oxyregulatory ability, calculated RI and hyperventilation response were consistent even when individuals of similar body sizes (for all but the smallest species, P. ovata, where there was no overlap in body mass range) were compared.
There was a significant effect of species on normoxic rates of O2 uptake. P. brevicornis had a significantly higher O2 uptake than either P. miersi or S. gracilis (table 1, electronic supplementary material, S2.2). The species-specific difference in mass standardized O2 uptake was driven by P. brevicornis alone (electronic supplementary material, S2.2).
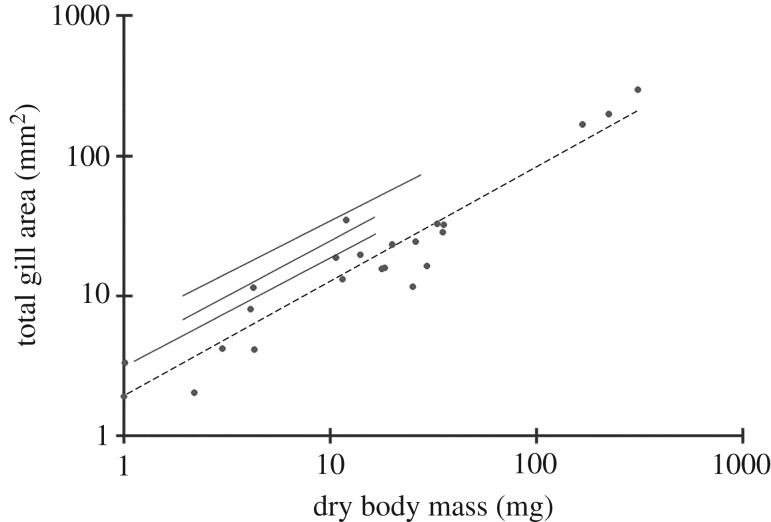
The total gill areas of P. miersi, P. brevicornis and S. gracilis all scaled with body mass and with a similar exponent to the three temperate marine gammarid species investigated by Moore & Taylor [38]; however, the Antarctic species had a consistently smaller mass-specific gill area (figure 3). There was no significant difference between the body mass–gill area relationships of the three species (ANCOVA F2,23 = 0.65, p = 0.53). If, however, the area of the putative EGS on the inside thin surfaces of the coxal plates of P. brevicornis is taken into account (electronic supplementary material, S4), the EGS would increase the total gas exchange surface of P. brevicornis by an average of 80% (range 65–128.3%) for an individual with a mean dry mass of 22 mg.
Figure 3.
Relationship between total gill area and dry body mass of three of the Antarctic amphipod species together with Moore & Taylor's [38] data on the gill areas of some temperate marine gammaridean amphipods. Dashed line = line of best fit (log10y = 0.813, log10x + 0.294, r2 = 90.52%). Each of the solid lines are lines of best fit for (from top to bottom) Echinogammarus pirloti, Gammarus locusta and Gammarus duebeni from Moore & Taylor [38].
(b). Putative respiratory pigment
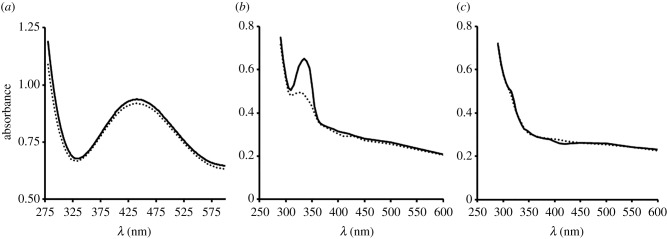
Presented in figure 4 are spectrophotometric scans of haemolymph from three of the four species: P. ovata proved too small to sample. A peak was detected at λ = 332 nm for haemolymph from P. brevicornis but not from S. gracilis. The peak disappeared when haemolymph was aspirated with nitrogen gas and partially reappeared when the haemolymph was aspirated with air, suggestive of reversible O2 binding to the Cu-bearing respiratory pigment haemocyanin (see electronic supplementary material, S5 for further details). A peak was detected at λ = 433 nm in P. miersi haemolymph but was unaltered when haemolymph was aspirated with nitrogen gas. This peak was identical to the carotenoids peak isolated from the brown seaweeds that this species regularly fed on (JIS 2007, unpublished data).
Figure 4.
Spectrophotometric scan of untreated haemolymph from three Antarctic amphipod species (a) P. miersi, (b) P. brevicornis and (c) S. gracilis. Solid line = air saturated. Broken line = deoxygenated.
(c). Crawling and swimming behaviour
All three species exhibited quite different crawling responses (F2,107 = 9.3, p = 0.003), with different responses to increasing temperature (F2,107 = 28.8, p < 0.001) (figure 5). Separate ANOVAs for each species followed by post hoc Tukey tests for each temperature showed that the crawling speed of P. brevicornis was faster at 0.1 and 3.1°C than at 6.5 and 9.6°C (T > 5.0, p < 0.001; figure 5a) and faster at 4.4°C than 9.6°C (T = 4.6, p < 0.001). The burst swimming speed of P. brevicornis was fastest at 3.1°C (T > 3.6, p < 0.008; figure 5b) but speeds also fell precipitously to be slowest at 6.5 and 9.6°C (T > 3.0, p < 0.037). Temperature significantly increased crawling speed of P. miersi (F4,53 = 3.2, p = 0.033) but crawling speed was not significantly different in pairwise comparisons (T < 2.8, p > 0.060). Crawling speed in S. gracilis was considerably slower, at about one third that of P. brevicornis and P. miersi, and did not change with temperature (F2,21 = 0.88, p = 0.43). All individuals of S. gracilis died at T = ∼6°C.
Figure 5.

Crawling (a) and burst swimming speed (b) of amphipods. Values are means ±1 s.d. Both crawling and swimming speeds were log10 transformed to normalize residuals (Anderson–Darling < 0.403, p > 0.333). Crawling speeds had equal variances (Levene's test, p > 0.058). (Online version in colour.)
4. Discussion
(a). Support for the oxygen limitation hypothesis
There is some support for the oxygen limitation hypothesis in our study. The largest species, P. miersi, did not perform as well (i.e. poor ability to maintain respiratory independence and mount a hypoxia-induced hyperventilatory response) as the smallest species Probolisca ovata under acutely declining PO2. The performance of the two intermediate-sized species lay between these two extremes. While there was no difference in the allometric relationship between total gill area and body size between the three species investigated, the total gas exchange surface of P. brevicornis increased markedly if EGS area was added to total gill area. That said, gill surface areas were consistently smaller (controlling for mass) than the only other aquatic amphipod species for which we have data, i.e. temperate shallow-water gammarids [38].
(b). Qualifications to support for the oxygen limitation hypothesis
The support that our study lends the oxygen limitation hypothesis requires qualification. First, P. brevicornis displayed a markedly better performance under hypoxia than the relatively closely related and slightly smaller S. gracilis, when controlling for body size. This better performance could be associated with (1) the best developed hypoxia-related ventilatory response measured; (2) putative EGS, which presumably markedly increase the total area available for gas exchange; and (3) the presence of the respiratory pigment haemocyanin, which carries and reversibly binds O2. Such innovations presumably enhance the ability of P. brevicornis to obtain and transport O2 to the tissues. Such a claim is strengthened by the fact that P. brevicornis has a markedly higher V̇O2 and is capable of considerably faster movement and swimming than similarly sized S. gracilis and P. miersi. So with respect to the prediction of the oxygen limitation hypothesis—that all else being equal, reduced O2 should have disproportionately larger effects on the performance of large-bodied individuals—this current work suggests that in the case of P. brevicornis all else is not equal. This species has respiratory adaptations likely linked with a comparatively good respiratory performance under declining PO2s and, compared with the other species studied, a very active lifestyle. Thus, it possesses a better respiratory performance than the oxygen limitation hypothesis would predict.
Second, there were only small improvements in respiratory performance in small individuals compared with larger individuals of the two largest species, P. miersi and P. brevicornis. These differences were not, however, statistically significant. These findings arguably lessen the support for the generality of the oxygen limitation hypothesis.
(c). Support for the symmorphosis hypothesis
As discussed, the respiratory performance and associated adaptations with respect to gas exchange and transport of P. brevicornis provide an exception to the oxygen limitation hypothesis. However, they do lend some support to the symmorphosis hypothesis [9]. The ‘exception’ of P. brevicornis fits with the prediction that effects of reduced O2 should be independent of body size: compared with S. gracilis, the respiratory system of P. brevicornis seems the subject of evolutionary change (development of EGS, and respiratory pigment) that could compensate for body size limitations. If true, P. brevicornis has been shaped to supply adequate O2 for metabolism in a species that, unlike the other three species, displays a very active lifestyle in what is generally considered a very sluggish environment. The principles of symmorphosis are also supported by the marked decline in swimming performance of P. bervicornis at temperatures above 3°C, which supports the notion that its respiratory system has evolved to maintain swimming speed in the constant cold of the Southern Ocean temperatures and cannot supply the demands of its active lifestyle at warmer temperatures. The fact that there was such a small (and non-significant) effect of mass on the respiratory performance of P. brevicornis and P. miersi under acutely declining O2, even over such a wide range of body sizes tested, could also be seen as supporting the notion of symmorphosis. Testing symmorphosis is notoriously difficult, but some of our findings are at least consistent with the hypothesis.
(d). Support for the respiratory advantage hypothesis
Our study also offers some support to the ‘respiratory advantage hypothesis' [10]. The largest, and arguably only ‘giant’, species we examined, P. miersi, had little or no oxyregulatory capacity, poor ventilatory ability showing little response to hypoxia and did not appear to have an O2-binding pigment like haemocyanin or haemoglobin dissolved in its haemolymph. This is consistent with the idea that large body size in an animal that does not have well-developed mechanisms for respiratory regulation bestows a respiratory advantage that can overcome viscosity-related, low O2 availability in polar waters and the prediction that gigantism should be absent in polar groups characterized by good respiratory control.
However, the second prediction, that reduced O2 should have a disproportionately large effect on the performance of small-bodied individuals gains little support from our study. Smaller individuals of P. miersi, equivalent in size to P. brevicornis and S. gracilis, did not have a significantly different oxyregulatory ability from (and had a similar ventilatory response to declining O2) to the largest individuals of the same species. Similarly, large individuals of P. brevicornis displayed the same respiratory performance under hypoxia as did smaller individuals.
(e). Conclusions and perspective
This study provides qualified support for the oxygen limitation hypothesis of Chapelle & Peck [8], but also some degree of support for the symmorphosis hypothesis [9] and perhaps even the counterintuitive respiratory advantage hypothesis [10].
We suggest that, on the basis of the support we found for the oxygen limitation hypothesis, giant polar amphipods are likely to be some of the first to fare badly in an O2-poor ocean. However, the products of past evolutionary innovation, such as respiratory pigments that enhance O2-transport and novel gas exchange structures in some if not all species, may, to some extent, offset any respiratory disadvantages of either large or small body size, consistent with the symmorphosis hypothesis. We suggest that the effects of O2 reductions on polar amphipods, and possibly other marine groups, depend on the composition of individual biologies and not just body size [21,25]. For example, knowledge of mechanisms comprising the respiratory biology of species, and perhaps even individuals (e.g. ventilation, perfusion, oxygen transport, cellular metabolism, anaerobic capacity) and how those mechanisms respond to, and can be induced by, environmental drivers is invaluable in understanding relationships between O2 and body size. This is particularly so for species that do not appear to have structures we naturally associate with particular functions, and yet the functions are present nevertheless, e.g. hypoxia-sensitive gut movements in Antarctic pycnogonids [32] and free-living brine shrimp embryos [39], which generate additional circulatory function helping to produce (or maintain) internal O2 diffusion gradients. So too is knowledge of factors affecting oxygen supply (e.g. water current, degree of hypoxia encountered in situ) and demand (scope for activity, behaviour of movement, feeding and growth rates). The key is to identify which factors are most important in terms of their influence on multi-species comparisons, and either incorporate or control for them.
In this present comparison, we are still left with the puzzle of why P. brevicornis should have evolved greater burst and crawling speed than the other species investigated, and how these innovations might be linked to respiratory innovations that appear to be absent from the other three. The explanation clearly lies in the natural history of the species, e.g. predation pressure, food acquisition. Currently, this natural history, as with much Antarctic life, is largely unknown.
It is tempting to assume that a greater number of closely related species (or at least species with a well-resolved phylogeny), encompassing a wide range of body sizes, on its own would have produced a stronger test of the predictions of the three hypotheses posited. However, this study has highlighted that it is not just number but the choice of species that is important. Treating species as statistically independent replicates, ignoring their phylogeny, has been shown (at least sometimes in physiological studies) to have its difficulties (e.g. [40]). However, ignoring the product of that evolutionary divergence, the resultant adaptations and convergence, as integrated into individual species is even more problematic. When testing macrophysiological hypotheses it may even lead to spurious patterns and imperfect tests of unifying hypotheses. Taking such products into account should instead strengthen such multi-species comparisons and meta-analyses.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Adam Burke, Theresa Murphy, Zachary Priestly, Sarah Reed, Ben Robinson and Kate Stanton for their assistance with amphipod collection, Ali Massey for her laboratory assistance, and Simon Rundle, Wilco Verbeck, Art Wood, Lloyd Peck and two anonymous referees for their critical comments on the manuscript.
Data accessibility
The datasets supporting this article will be uploaded as part of the electronic supplementary material.
Authors' contributions
Both authors contributed to the conception and design of the experiments, and the acquisition, analysis and interpretation of the data. Both authors contributed to drafting, revising and approving the submitted manuscript.
Competing interests
We declare we have no competing interests.
Funding
J.I.S. was supported by an NERC CASS award (no. 124) and S.A.M. was supported by NERC core funding to the British Antarctic Survey's ‘Biodiversity, Adaptation and Evolution’ Team.
References
- 1.Pörtner HO, Karl DM, Boyd PW, Cheung WWL, Lluch-Cota SE, Nojiri Y, Schmidt DN, Zavialov PO. 2014. Ocean systems. In Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change (eds Field CB, et al.), pp. 411–484. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Hillebrand H, Brey T, Gutt J, Hagen W, Metfies K, Meyer B, Lewandowska A. 2018. Climate change: warming impacts on marine biodiversity. In Handbook on marine environment protection, pp. 353–373. Berlin, Germany: Springer. [Google Scholar]
- 3.Miller DD, Ota Y, Sumaila UR, Cisneros-Montemayor AM, Cheung WW. 2018. Adaptation strategies to climate change in marine systems. Glob. Change Biol. 24, e1–e14. ( 10.1111/gcb.13829) [DOI] [PubMed] [Google Scholar]
- 4.Somero GN.2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 5.Bozinovic F, Pörtner HO. 2015. Physiological ecology meets climate change. Ecol. Evol. 5, 1025–1030. ( 10.1002/ece3.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonan GR, Doney SC. 2018. Climate, ecosystems, and planetary futures: the challenge to predict life in Earth system models. Science 359, eaam8328 ( 10.1126/science.aam8328) [DOI] [PubMed] [Google Scholar]
- 7.Breitburg D, et al. 2018. Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 ( 10.1126/science.aam7240) [DOI] [PubMed] [Google Scholar]
- 8.Chapelle G, Peck LS. 1999. Polar gigantism dictated by oxygen availability. Nature 399, 114–115. ( 10.1038/20099) [DOI] [Google Scholar]
- 9.Woods HA, Moran AL, Arango CP, Mullen L, Shields C. 2008. Oxygen hypothesis of polar gigantism not supported by performance of Antarctic pycnogonids in hypoxia. Proc. R. Soc. B 276, 1069–1075. ( 10.1098/rspb.2008.1489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verberk WC, Atkinson D. 2013. Why polar gigantism and Palaeozoic gigantism are not equivalent: effects of oxygen and temperature on the body size of ectotherms. Funct. Ecol. 27, 1275–1285. ( 10.1111/1365-2435.12152) [DOI] [Google Scholar]
- 11.Ferrón HG, Martínez-Pérez C, Botella H. 2018. The evolution of gigantism in active marine predators. Historic. Biol. 30, 712–716. ( 10.1080/08912963.2017.1319829) [DOI] [Google Scholar]
- 12.Harrison JF. 2017. Do performance–safety trade-offs cause hypometric metabolic scaling in animals? Trends Ecol. Evol. 32, 653–664. ( 10.1016/j.tree.2017.05.008) [DOI] [PubMed] [Google Scholar]
- 13.Audzijonyte A, Barneche DR, Baudron AR, Belmaker J, Clark TD, Marshall CT, Morrongiello JR, van Rijn I. 2018. Is oxygen limitation in warming waters a valid mechanism to explain decreased body sizes in aquatic ectotherms? Glob. Ecol. Biogeogr. 28, 64–77. ( 10.1111/geb.12847) [DOI] [Google Scholar]
- 14.Verberk WC, Leuven RS, van der Velde G, Gabel F. 2018. Thermal limits in native and alien freshwater peracarid Crustacea: the role of habitat use and oxygen limitation. Funct. Ecol. 32, 926–936. ( 10.1111/1365-2435.13050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Broyer C. 1977. Analysis of the gigantism and dwarfness of Antarctic and Subantarctic Gammaridean amphipoda. In Adaptations within Antarctic ecosystems. Proc. 3rd SCAR (ed. Llano GA.), pp. 327–334. Washington, DC: Symposium. Antarctic Biology, Smithsonian Institution. [Google Scholar]
- 16.Peck LS, Chapelle G. 2003. Reduced oxygen at high altitude limits maximum size. Proc. R Soc. B 270(Suppl 2), S166–S167. ( 10.1098/rsbl.2003.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapelle G, Peck LS. 2004. Amphipod crustacean size spectra: new insights in the relationship between size and oxygen. Oikos 106, 167–175. ( 10.1111/j.0030-1299.2004.12934.x) [DOI] [Google Scholar]
- 18.McClain CR, Rex MA. 2001. The relationship between dissolved oxygen concentration and maximum size in deep-sea turrid gastropods: an application of quantile regression. Mar. Biol. 139, 681–685. ( 10.1007/s002270100617) [DOI] [Google Scholar]
- 19.Peck LS, Morley SA, Pörtner HO, Clark MS. 2007. Thermal limits of burrowing capacity are linked to oxygen availability and size in the Antarctic clam Laternula elliptica. Oecologia 154, 479–484. ( 10.1007/s00442-007-0858-0) [DOI] [PubMed] [Google Scholar]
- 20.Woods HA, Moran AL, Tobalske BW, Lane SJ, Shishido CL. 2016. Temperature–oxygen interactions and the evolution of giant Antarctic sea spiders. FASEB J. 30(1_supplement), 1230–1231. [Google Scholar]
- 21.Spicer JI, Gaston KJ. 1999. Amphipod gigantism dictated by oxygen availability? Ecol. Lett. 2, 397–403. ( 10.1046/j.1461-0248.1999.00105.x) [DOI] [Google Scholar]
- 22.Lane SJ, Shishido CM, Moran AL, Tobalske BW, Arango CP, Woods HA. 2017. Upper limits to body size imposed by respiratory–structural trade-offs in Antarctic pycnogonids. Proc. R. Soc. B 284, 20171779 ( 10.1098/rspb.2017.1779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane SJ, Moran AL, Shishido CM, Tobalske BW, Woods HA. 2018. Cuticular gas exchange by Antarctic sea spiders. J. Exp. Biol. 221, jeb177568 ( 10.1242/jeb.177568) [DOI] [PubMed] [Google Scholar]
- 24.Weibel ER, Taylor CR, Hoppeler H. 1991. The concept of symmorphosis: a testable hypothesis of structure–function relationship. Proc. Natl Acad. Sci. USA 88, 10 357–10 361. ( 10.1073/pnas.88.22.10357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran AL, Woods HA. 2012. Why might they be giants? Towards an understanding of polar gigantism. J. Exp. Biol. 215, 1995–2002. ( 10.1242/jeb.067066) [DOI] [PubMed] [Google Scholar]
- 26.Verberk WCEP, Bilton DT, Calosi P, Spicer JI. 2011. Oxygen supply in aquatic ectotherms: partial pressure and solubility together explain biodiversity and size patterns. Ecology 92, 1565–1572. ( 10.1890/10-2369.1) [DOI] [PubMed] [Google Scholar]
- 27.Angilletta MJ, Steury TD, Sears MW. 2004. Temperature, growth rate and body size in ectotherms: fitting pieces of a life-history puzzle. Integr. Comp. Biol. 44, 498–509. ( 10.1093/icb/44.6.498) [DOI] [PubMed] [Google Scholar]
- 28.Spicer JI. 2016. Respiratory responses of marine animals to environmental hypoxia. In Stressors in the marine environment. Physiological and ecological responses; societal implications, Chapter 2 (eds Solan M, Whiteley NM), pp. 25–35. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Cussans M. 1904. Gammarus XII. In L.M.B.C. Memoirs on typical British marine plants and animals (ed. Herdman WA.). London, UK: Williams and Norgate. [Google Scholar]
- 30.Hudson LJ, Maitland DP. 1996. Anatomy of structures associated with air-breathing in Orchestia gammarellus (Crustacea: Amphipoda: Talitridae): coxal plates and gills. Mar. Biol. 125, 287–295. ( 10.1007/BF00346309) [DOI] [Google Scholar]
- 31.Peck LS. 2018. Antarctic marine biodiversity: adaptations, environments and responses to change. Oceanogr. Mar. Biol. Annu. Rev. 56, 105–236. ( 10.1201/9780429454455-3) [DOI] [Google Scholar]
- 32.Woods HA, Lane SJ, Shishido C, Tobalske BW, Arango CP, Moran AL. 2017. Respiratory gut peristalsis by sea spiders. Curr. Biol. 27, R638–R639. ( 10.1016/j.cub.2017.05.062) [DOI] [PubMed] [Google Scholar]
- 33.Truebano M, Tills O, Collins M, Clarke C, Shipsides E, Wheatley C, Spicer JI. 2018. Short-term acclimation in adults does not predict offspring acclimation potential to hypoxia. Sci. Rep. 8, 3174 ( 10.1038/s41598-018-21490-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agnew DJ, Taylor AC. 1985. The effect of oxygen tension on the physiology and distribution of Echinogammarus pirloti (Sexton & Spooner) and E. obtusatus (Dahl) (Crustacea: Amphipoda). J. Exp. Mar. Biol. Ecol. 87, 169–190. ( 10.1016/0022-0981(85)90089-9) [DOI] [Google Scholar]
- 35.Mueller CA, Seymour RS. 2011. The regulation index: a new method for assessing the relationship between oxygen consumption and environmental oxygen. Physiol. Biochem. Zool. 84, 522–532. ( 10.1086/661953) [DOI] [PubMed] [Google Scholar]
- 36.Marshall DJ, Bode M, White CR. 2013. Estimating physiological tolerances – a comparison of traditional approaches to nonlinear regression techniques. J. Exp. Biol. 216, 2176–2182. ( 10.1242/jeb.085712) [DOI] [PubMed] [Google Scholar]
- 37.Lagos ME, Barneche DR, White CR, Marshall DJ. 2017. Do low oxygen environments facilitate marine invasions? Relative tolerance of native and invasive species to low oxygen conditions. Glob. Change Biol. 23, 2321–2330. ( 10.1111/gcb.13668) [DOI] [PubMed] [Google Scholar]
- 38.Moore PG, Taylor AC. 1984. Gill area relationships in an ecological series of gammaridean amphipods (Crustacea). J. Exp. Mar. Biol. Ecol. 74, 179–186. ( 10.1016/0022-0981(84)90085-6) [DOI] [Google Scholar]
- 39.Spicer JI. 2006. Gut reaction by heartless shrimps: experimental evidence for the role of the gut in generating circulation before cardiac ontogeny. Biol. Lett. 2, 580–582. ( 10.1098/rsbl.2006.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond SE, Chick LD. 2018. The Janus of macrophysiology: stronger effects of evolutionary history, but weaker effects of climate on upper thermal limits are reversed for lower thermal limits in ants. Curr. Zool. 64, 223–230. ( 10.1093/cz/zox072) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article will be uploaded as part of the electronic supplementary material.