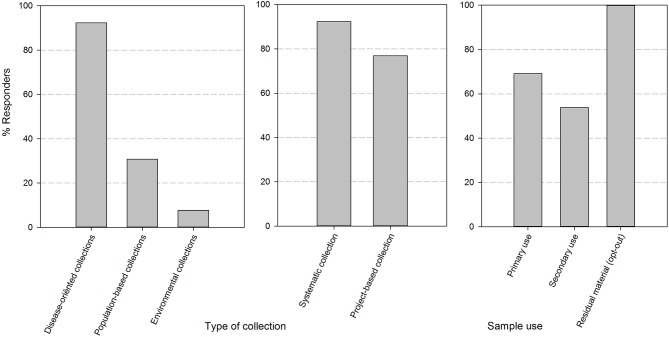
Figure 1.
The set-up and composition of human body material collections in biobanks of the BBMRI.be network. Responses were divided by the nature of the human body material collection (collection with a disease orientation, population-based or on environmental ground), by the method of collection of the material (systematic i.e., by sampling at occasions within the clinical path of the patient or project-based i.e., by sampling in the framework of a specific study/project at pre-defined and study-based time points) and by the type of use as defined in the Belgian legislation (primary use: the use to which the donor has explicitly consented to at the time of collection; secondary use: any other use than the one consented to at the time of collection; residuary material: the portion of human bodily material that has been taken from the donor for diagnostic or treatment purposes which, when a sufficient sample is safeguarded for refinement or completion of these purposes, is superfluous to these purposes and as such could be destroyed—presumed consent applies).