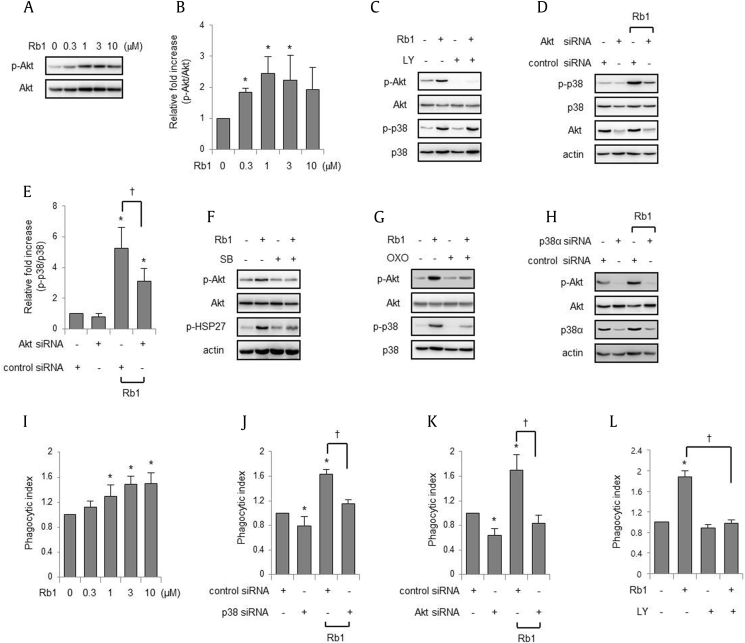
Fig. 3.
Rb1 increases the phosphorylation of Akt through the p38 MAPK pathway. (A–B) Peritoneal macrophages were cultured with Rb1 for 1 h at the indicated doses. The cell lysates were subjected to western blotting. Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control. (C, F, and G) Macrophages were cultured with LY294002 (LY; 0 or 10 μM) for 30 min, SB203580 (SB; 0 or 10 μM) for 30 min or (5Z)-7-oxozeaenol (OXO; 0 or 1 μM) for 1 h before exposure to Rb1 (0 or 3 μM) for 1 h. (D–E) RAW 264.7 cells were treated with control small-interfering RNA (siRNA) or siRNA targeting mouse Akt and cultured with Rb1 (0 or 3 μM) for 1 h. Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control siRNA; †p < 0.05. (H) RAW 264.7 cells were treated with control or p38α siRNA and cultured with Rb1 (0 or 3 μM) for 1 h. The whole cell lysates were subjected to electrophoresis in polyacrylamide gels. Representative gels are shown, and the other two independent experiments provided similar results. (I) RAW 264.7 cells were cultured with Rb1 (0, 0.3, 1, 3, and 10 μM) for 2 h and subjected to FITC-conjugated E. coli for 30 min, followed by flow cytometry. (J–K) RAW 264.7 cells were treated with control siRNA or siRNA targeting mouse p38α or Akt. The cells were cultured with Rb1 (0 or 3 μM) for 2 h and subjected to FITC-conjugated E. coli for 30 min, followed by flow cytometry. (L) LY294002 (LY; 0 or 10 μM) was added to peritoneal macrophage culture for 30 min before exposure to Rb1 (0 or 3 μM) for 2 h, and FITC-conjugated E. coli was added to the culture media for 20 min, followed by flow cytometry. Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control; †p < 0.05. FITC, fluorescein isothiocyanate; MAPK, mitogen-activated protein kinase; SD, standard deviation.