Figure 1. CDK1/2‐dependent phosphorylation of NICD links the cell cycle and the segmentation clock.
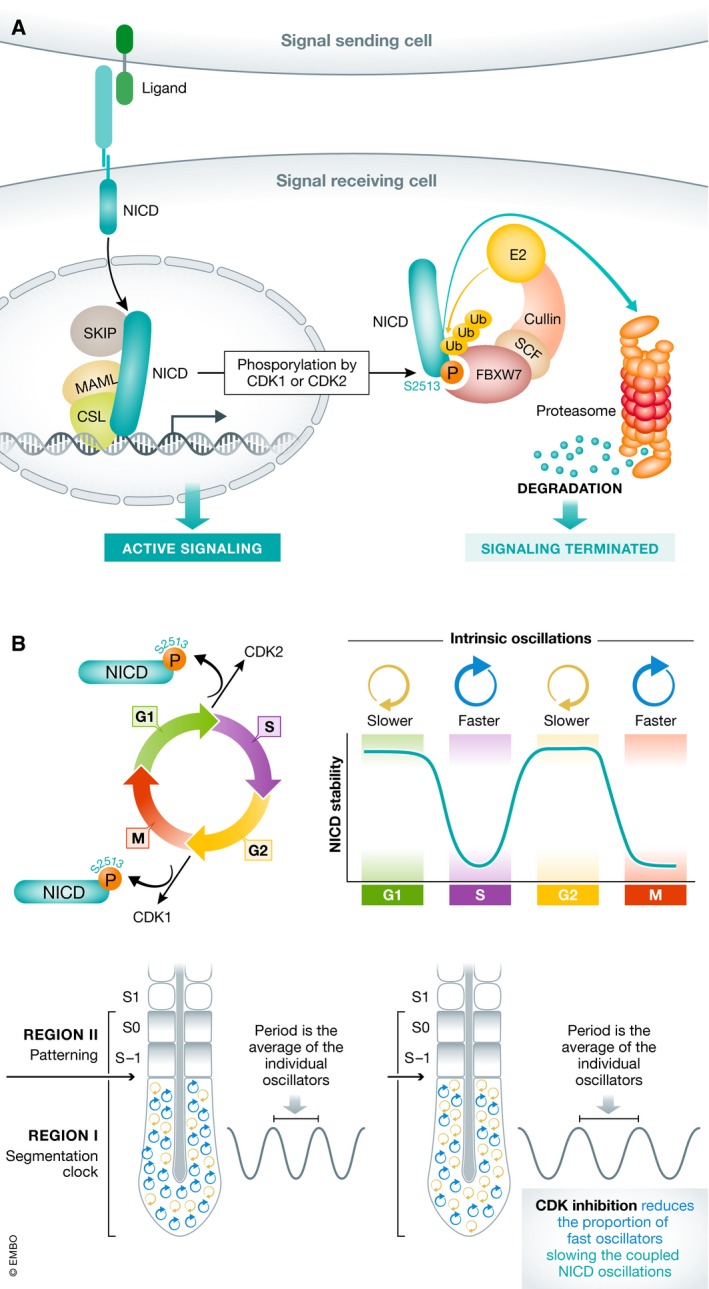
(A) Binding of a ligand on a signal‐sending cell to a receptor on a signal‐receiving cell releases NICD to enter the nucleus of the signal‐receiving cell, bind DNA, and promote active signaling. Phosphorylation of S2513 promotes FBXW7 binding and promotes termination of signal by targeting NICD for SCF E3 ubiquitin ligase‐dependent proteasomal degradation. (B) Peaks of CDK1 or CDK2 activity alter NICD stability in a cell cycle‐dependent manner. If NICD stability influences the intrinsic period of the cell autonomous oscillator, then the cell cycle position of cells in the PSM influences the period of synchronized segmentation clock oscillations. Treatments that block CDK1 activity in the PSM are predicted to reduce the proportion of fast oscillators, slowing the tissue‐level oscillations.
