Abstract
The role of tumor-infiltrating lymphocytes (TILs) suggests that cancer is a disease with not only a genetic, but also an immunological basis. Additionally, immune cell infiltration is an important feature of oral cancer. C-X-C motif chemokine ligand 12 (CXCL12) serves an important role in immune suppression in the tumor microenvironment. Therefore, the present study investigated how CXCL12 expression in oral squamous cell carcinoma (OSCC) was associated with clinicopathological parameters and TILs distribution. Complete CXCL12, TIL and clinical data were available for 45 patients with oral cancer treated by surgery. Expression levels of CXCL12, CD8+ TILs and forkhead box P3 (FoxP3+) TILs were assessed by immunohistochemistry in OSCC samples. CXCL12 expression in OSCC cells was observed in 32 (68.9%) cases and was associated with poor differentiation (P=0.045), advanced stages (P<0.001), tumor recurrence (P=0.011), poor overall survival (P=0.0476) and a higher density of FoxP3+ TILs (P<0.001). The CD8+/FoxP3+ ratio was lower in patients with poor differentiation (P=0.034), advanced stage tumors (P=0.015) and tumor recurrence (P=0.002). In addition, the ratio of CD8+/FoxP3+ TILs was significantly associated with the 5-year overall survival rate (P<0.001). The CD8+/FoxP3+ ratio was indicated to be a stronger prognostic indicator compared with the density of FoxP3+ TILs or CD8+ TILs. The present study identified an association between increased CXCL12 expression and FoxP3+ cell infiltration in OSCC. Targeting the CXCL12/C-X-C motif chemokine receptor 4 axis in OSCC may be employed as a novel strategy of tumor immunotherapy in the future.
Keywords: C-X-C motif chemokine ligand 12, tumor-infiltrating lymphocytes, cluster of differentiation 8, forkhead box P3
Introduction
Oral squamous cell carcinoma (OSCC) accounts for >90% of primary oral malignancies worldwide (1). A combination of different genetic features and environmental factors contributes to the oncogenesis of OSCC (2). Although treatment methods, including chemotherapy, radiotherapy and surgery, have advanced, the 5-year overall survival rate of patients with OSCC remains ~50% (3). Locoregional recurrence, metastatic disease and second primary tumors are the main factors contributing to the poor survival rate of patients with OSCC (4).
OSCCs are often ulcerated with a large number of lymphocyte infiltration. An improved understanding of the tumor immune microenvironment, including number, location and function of infiltrating lymphocytes is crucial in order to examine and test immunotherapeutic strategies, which may prolong the survival time of patients with OSCC (5). Tumor-infiltrating lymphocytes (TILs) are considered to be the host immune response to tumor cells (6). To date, a number of studies have reported an association between the TILs subset and patient prognosis in various types of cancer (6,7). Tumor infiltration by CD8+ T cells, including cytotoxic T cells (CTLs), has been indicated to be associated with an improved prognosis in various types of malignant tumors (7). In turn, increasing evidence has demonstrated that tumor-infiltrating forkhead box P3 (FoxP3)+ immunosuppressive regulatory T cells (Tregs) are associated with poor prognosis, and suppressed function on host antitumor immunity (8). The ratio of cytotoxic CD8+ T cells and FoxP3+ Tregs in the tumor microenvironment has been indicated to be a prognostic factor in in various types of cancer (9–11). However, further investigations on the role of TILs in OSCC are required.
C-X-C motif chemokine ligand 12 (CXCL12) serves multiple tumor promoting functions via its cognate receptor C-X-C motif chemokine receptor (CXCR)4 present in cancer cells; either directly, by enhancing tumor growth, migration and invasiveness, or indirectly, by recruiting endothelial progenitors required for tumor angiogenesis (12,13). In addition, CXCL12 promotes tumor immunosuppression by recruiting specific immune cell populations (13). Therefore, it may be hypothesized that the expression levels of CXCL12 in OSCC are associated with tumor progression and immune suppression. To examine this hypothesis, the present study investigated the association among clinicopathological parameters of OSCC and CXCL12, densities of CD8+ T cells and FoxP3+ T cells. In addition, the present study examined whether CXCL12 expression can influence CD8+ T cells or FoxP3+ T cells distribution in patients with OSCC.
Patients and methods
Patients and sample collection
The present study was performed on a retrospective cohort of 45 Chinese patients with primary OSCC. A total of 24 patients were male and 21 were female, and the median age was 61 (range, 37–81 years). Paraffin-embedded tissue specimens, including OSCC tissue and adjacent non-cancerous tissue, were collected from the West China College of Stomatology, Sichuan University between January 2011 and December 2011. All patients underwent radical surgery with neck dissection without receiving any preoperative radiotherapy and chemotherapy. All patients signed informed consent forms prior to the present study. Data from patient follow-up and clinicopathological characteristics were collected from the database of West China College of Stomatology and telephone interviews. Clinical staging was established according to the American Joint Committee on Cancer Staging Manual (2009) (14). The present study was approved by the Institutional Review Board of West China Hospital of Stomatology of Sichuan University.
Immunohistochemistry
Formalin-fixed at 4°C for 24 h, paraffin-embedded tissues, obtained from the Department of Pathology, West China Hospital of Stomatology, were consecutively cut into 5-µm sections and transferred onto silanized glass slides. Immunohistochemistry for CXCL12, CD8 and FoxP3 was performed using standard procedures (8,9). Briefly, 5-µm tissue sections were dewaxed and rehydrated using xylene and graded alcohol washes. The slides were heated to 121°C for 2 min for antigen retrieval in citrate buffer (pH 6.0). Following serial blocking with 3% hydrogen peroxide and 5% normal goat serum (cat. no., ZLI-9022; OriGene Technologies, Inc.) at 37°C for 20 min, the sections were incubated with primary monoclonal antibody against CD8 (dilution, 1:100; clone C8/144B; Dako), CXCL12 (dilution, 1:400; cat. no. ab9797; Abcam) or FoxP3 (dilution 1:100; cat. no. ab20034; Abcam) for 16 h at 4°C. The sections were then incubated with biotinylated goat anti-mouse immunoglobulin and peroxidase-conjugated streptavidin (dilution, 1:100; cat. no., cat. no., ab64255; Abcam) at 37°C for 30 min. The enzyme substrate was 3,3′-diaminobenzidine tetrahydrochloride. Tissue section were analyzed using a light microscope. Negative controls were performed with PBS instead of the primary antibody.
Evaluation of immunohistochemistry results
For CXCL12 expression, the respective score was calculated according to a visual grading scale based on the percentage of positive cells and the intensity of staining. The percentage scale was as follows: 0, <5; 1, 5–25; 2, 25–50; 3, 50–75; and 4, >75%. The intensity scale was as follows: 0, none; 1, weak staining; 2, moderate staining; and 3, strong staining. Five representative fields of view were evaluated (magnification, ×400). The final weighted score was calculated for each case by multiplying the two scores: <1, negative; ≥1, positive (15). The patients were divided into high and low expression CXCL12 groups according to the median values.
For CD8+ T and FoxP3+ T cells, tissue sections were examined microscopically (magnification, ×400), five high-power fields of each case with the most abundant TILs were selected for image capturing at an area of 0.0625 mm2, and the numbers of CD8+ T and FoxP3+ T cells in each field were counted in the same five areas of serial sections. The mean value represented the number of lymphocyte infiltration, as described previously (16).
Statistical analysis
The data was presented as mean ± standard deviation. For CXCL12 expression and TILs densities, five areas were measured and the mean score of the five areas was collected for further analyses. Associations among clinicopathological features, CXCL12 expression and densities of TILs were evaluated using the χ2 test. The variables were dichotomized via median splits. Survival curves were plotted using the Kaplan-Meier method, and significant differences in the overall survival rate were assessed using a log-rank test. The correlation between variables was calculated by linear regression. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS version 19.0 (IBM Corp.).
Results
Characteristics of patients with OSCC included in the present study
Between January 2011 and December 2011, a total of 45 specimens were collected from 45 pathologically confirmed patients with OSCC. Out of these patients, 24 were male, 29 had advanced disease, 14 exhibited poor differentiation and 19 exhibited positive lymph node metastasis.
General features of TILs in OSCC
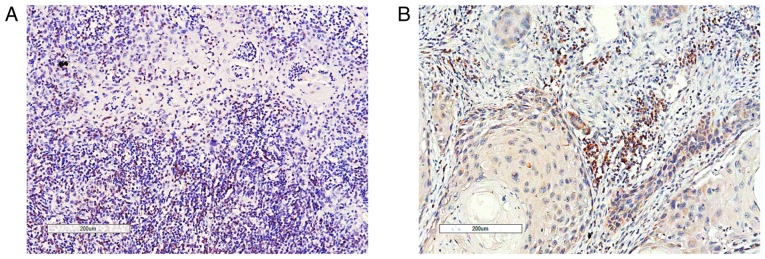
TILs were distributed in the cancer cell nests and in the stroma of the tumor-host interface. The majority of FoxP3+ T cells were located in the stroma (Fig. 1B). However, CD8+ T cells infiltrated not only the stroma of the tumor-host interface but also the cancer cell nests (Fig. 1A). CD8+ TILs and FoxP3+ TILs were present in every case, with an average number of 111.9 and 50.4 per 0.0625 mm2, respectively (Table I). The median value of the CD8+/FoxP3+ ratio was 2.07, ranging between 0.1 and 20 (data not shown).
Figure 1.
Representative images of tumor-infiltrating lymphocytes in oral squamous cell carcinoma. (A) CD8+ T cells which infiltrated the cancer nest and stroma. (B) Forkhead box P3+ T cells. Infiltration was mostly confined to the cancer stroma.
Table I.
Expressions levels of FoxP3 and CD8 in TILs in oral squamous cell carcinoma.
| Variables | FoxP3+ TIL | CD8+ TIL |
|---|---|---|
| Number of patients, n | 45 (100%) | 45 (100%) |
| Average number per 0.0625 mm2, n | 50.4 | 111.9 |
| Range | 9–99 | 7–259 |
| Location | Stroma | Stroma |
FoxP3, forkhead box P3; TIL, tumor-infiltrating lymphocyte.
Expression levels of CXCL12 in OSCC cells
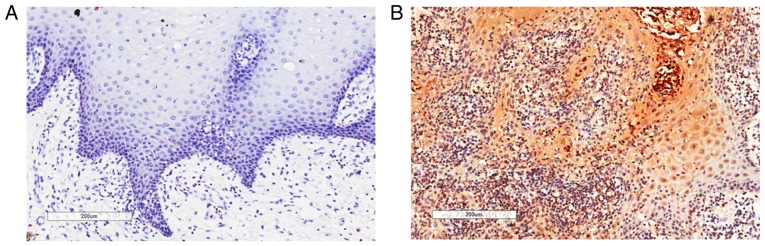
Among the available specimens, 11 specimens included OSCC tissues and adjacent non-cancerous tissues (epithelia and stroma). The non-cancerous tissues were collected from 0.5–1 cm apart from the margin. These samples were selected to compare CXCL12 expression between tumor adjacent tissues and OSCC tissues. The present study demonstrated that, in these specimens, CXCL12 could not be detected in adjacent non-cancerous tissues (Fig. 2A). CXCL12 expression in OSCC cells was observed in 68.9% (31/45) of OSCC cases. Cytoplasmic and intracellular staining patterns were observed in OSCC tissues (Fig. 2B).
Figure 2.
Representative images of immunohistochemical CXCL12 staining. (A) CXCL12 immunostaining in human normal epithelia. (B) Positive expression of CXCL12 in oral squamous cell carcinoma samples. CXCL12, C-X-C motif chemokine ligand 12.
Association among TILs density in the tumor microenvironment and clinicopathological features/survival of patients with OSCC
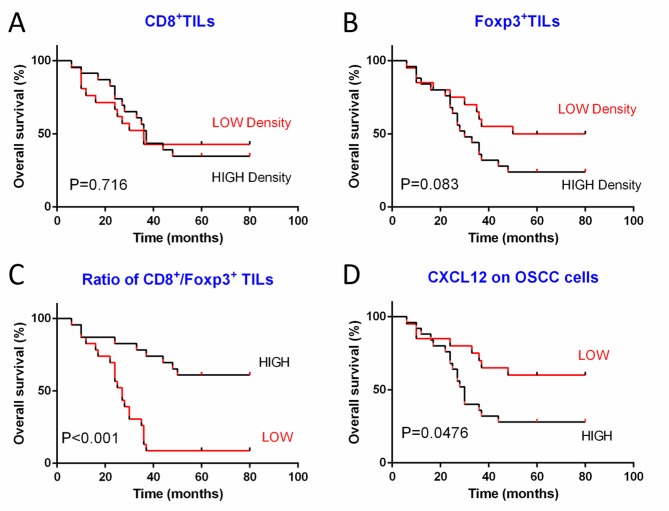
Associations among clinicopathological features and TILs density are summarized in Table II. An increased number of FoxP3+ TILs was associated with tumor recurrence (P=0.022) in patients with OSCC. There was no significant association identified among the density of CD8+ TILs and any of the clinicopathological features of patients with OSCC examined in the present study. Furthermore, neither the density of CD8+ TILs nor the density of FoxP3+ TILs were associated with patient survival (Fig. 3A and B). However, the ratio of these two types of TILs was suggested to be more important compared with their densities when evaluating the clinicopathological features and survival of patients (9). In particular, a low CD8+/FoxP3+ ratio was significantly associated with poor differentiation (P=0.034), advanced stage tumors (P=0.015) and tumor recurrence (P=0.002). In addition, low CD8+/FoxP3+ TILs ratios were associated with a poor 5-year overall survival rate (P<0.001; Fig. 3C).
Table II.
Associations between clinicopathological parameters of patients with oral squamous cell carcinoma and densities of TILs.
| FoxP3+ TIL | CD8+ TIL | CD8/FoxP3 ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Number, n | Low | High | P-value | Low | High | P-value | Low | High | P-value |
| Age | 0.174 | 0.214 | 0.140 | |||||||
| ≤59 | 24 | 12 | 12 | 11 | 13 | 10 | 14 | |||
| >59 | 21 | 8 | 13 | 11 | 10 | 12 | 9 | |||
| Sex | 0.231 | 0.232 | 0.178 | |||||||
| Male | 24 | 11 | 13 | 12 | 12 | 13 | 11 | |||
| Female | 21 | 9 | 12 | 10 | 11 | 9 | 12 | |||
| Tumor size | 0.165 | 0.233 | 0.142 | |||||||
| T1, T2 | 19 | 7 | 12 | 9 | 10 | 11 | 8 | |||
| T3, T4 | 26 | 13 | 13 | 13 | 13 | 11 | 15 | |||
| Lymph node metastasis | 0.230 | 0.142 | ||||||||
| Positive | 19 | 8 | 11 | 11 | 8 | 12 | 7 | 0.065 | ||
| Negative | 26 | 12 | 14 | 11 | 15 | 10 | 16 | |||
| Stage (14) | 0.125 | 0.132 | 0.015a | |||||||
| I, II | 16 | 9 | 7 | 6 | 10 | 4 | 12 | |||
| III, IV | 29 | 11 | 18 | 16 | 13 | 18 | 11 | |||
| Differentiation | 0.095 | 0.251 | 0.034a | |||||||
| Well-moderate | 31 | 16 | 15 | 15 | 16 | 12 | 19 | |||
| Poor | 14 | 4 | 10 | 7 | 7 | 10 | 4 | |||
| Recurrence | 0.022a | 0.097 | 0.002a | |||||||
| Yes | 24 | 7 | 17 | 14 | 10 | 17 | 7 | |||
| No | 21 | 13 | 8 | 8 | 13 | 5 | 16 | |||
FoxP3, forkhead box P3; TIL, tumor-infiltrating lymphocyte. P<0.05 used to indicate statistical significance.
The difference is significant.
Figure 3.
Survival analyses of 45 patients with OSCC. Kaplan-Meier plots with subsequent log-rank test results for (A) density of CD8+ TILs, (B) density of FoxP3+ TILs, (C) ratio of CD8+/FoxP3+ TILs and (D) CXCL12 expression in tumor cells. CXCL12, C-X-C motif chemokine ligand 12; FoxP3, forkhead box P3; OSCC, oral squamous cell carcinoma; TIL, tumor-infiltrating lymphocyte.
Association between CXCL12 expression and clinicopathological features/survival of patients with OSCC
As indicated in Table III, patients with poor differentiation (P=0.045), advanced stage tumors (P<0.001) and tumor recurrence (P=0.011) tended to exhibit a higher CXCL12 expression level. No significant associations among CXCL12 expression and lymph node metastasis (P=0.200) and tumor size (P=0.200) were identified. In addition, high CXCL12 expression was associated with poor overall survival (P=0.0476; Fig. 3D).
Table III.
Associations between CXCL12 expression and clinicopathological parameters of patients with oral squamous cell carcinoma and density of TILs.
| CXCL12 in tumor cells | ||||
|---|---|---|---|---|
| Variable | Number, n | Low score, n | High sore, n | P-value |
| Age | 0.056 | |||
| ≤59 | 24 | 13 | 11 | |
| >59 | 21 | 6 | 15 | |
| Sex | 0.189 | |||
| Male | 24 | 9 | 15 | |
| Female | 21 | 10 | 11 | |
| Tumor size | 0.200 | |||
| T1, T2 | 19 | 7 | 12 | |
| T3, T4 | 26 | 12 | 14 | |
| Lymph node metastasis | 0.200 | |||
| Positive | 19 | 7 | 12 | |
| Negative | 26 | 12 | 14 | |
| Stage (14) | <0.001a | |||
| I, II | 16 | 12 | 4 | |
| III, IV | 29 | 7 | 22 | |
| Differentiation | 0.045a | |||
| Well-moderate | 31 | 16 | 15 | |
| Poor | 14 | 3 | 11 | |
| Recurrence | 0.011a | |||
| Yes | 24 | 6 | 18 | |
| No | 21 | 13 | 8 | |
| FoxP3+ TIL | <0.001a | |||
| Low | 20 | 15 | 5 | |
| High | 25 | 4 | 21 | |
| CD8+ TIL | 0.095 | |||
| Low | 22 | 7 | 15 | |
| High | 23 | 12 | 11 | |
| CD8/FoxP3 ratio | <0.001a | |||
| Low | 22 | 2 | 20 | |
| High | 23 | 17 | 6 | |
CXCL12, C-X-C motif chemokine ligand 12; FoxP3, forkhead box P3; TIL, tumor-infiltrating lymphocyte. P<0.05 used to indicate statistical significance.
The difference is significant.
Association between CXCL12 expression and TILs density
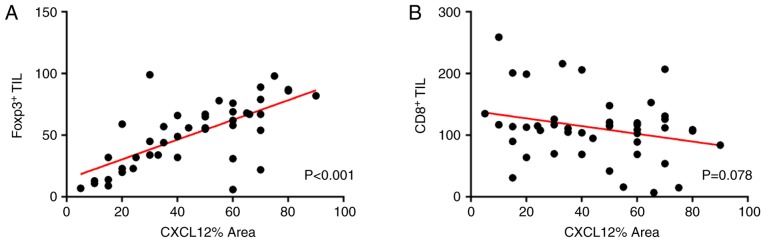
The density of FoxP3+ TILs exhibited a significant positive association with the CXCL12 expression of tumor cells (P<0.001). However, the density of CD8+ TILs was not identified to be associated with CXCL12 expression (P=0.095). In addition, the ratio of CD8+/FoxP3+ exhibited a significant negative association with CXCL12 expression (P<0.001; Table III).
To investigate the association between TILs and CXCL12 expression, a linear regression test was performed. The results indicated a correlation between CXCL12 percentage area and the density of FoxP3+ TILs (R2=0.481, P<0.001; Fig. 4A). By contrast, the CD8+ TILs were not identified to be significantly correlated with CXCL12 percentage area (R2=0.070, P=0.078; Fig. 4B). Further observations revealed that the higher the expression level of CXCL12, the larger the number of FoxP3+ T-cells infiltrating in the tumor microenvironment (Fig. 5A and B). Conversely, FoxP3+ T-cells hardly infiltrated in the region with low CXCL12 expression (Fig. 5A and B).
Figure 4.
Correlation between CXCL12% area and two TIL subpopulations. (A) Correlation of CXCL12% area with FoxP3+ TILs was indicated to be statistically significant (R2=0.481, P<0.001). (B) No statistically significant correlation of CXCL12% area with CD8+ TILs was observed (R2=0.070, P=0.078). The linear regression line is plotted in each case. CXCL12, C-X-C motif chemokine ligand 12; FoxP3, forkhead box P3; TIL, tumor-infiltrating lymphocyte.
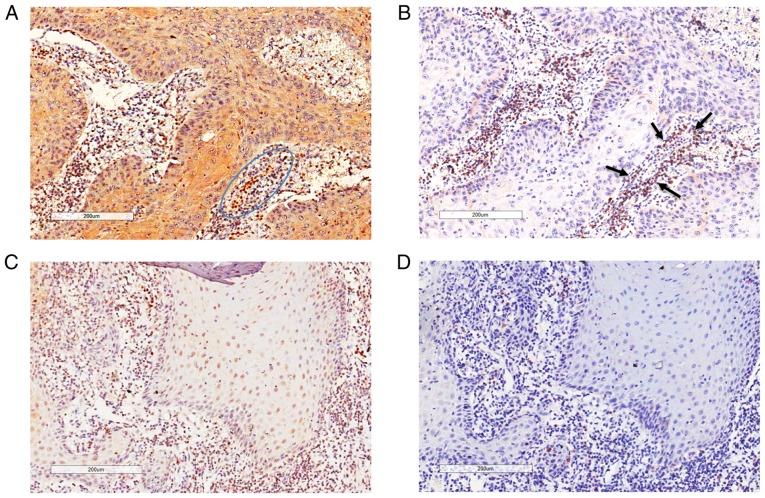
Figure 5.
CXCL12 may influence the distribution of FoxP3+ TILs. (A) High CXCL12 expression region. (B) Large number of FoxP3+ TILs infiltrating stroma. (A and B) Stroma cells with high CXCL12 expression (blue circle) were surrounded by numerous FoxP3+ TILs (black arrows). (C) Low CXCL12 expression region. (D) Small number of FoxP3+ TILs infiltrating stroma. CXCL12, C-X-C motif chemokine ligand 12; FoxP3, forkhead box P3; TIL, tumor-infiltrating lymphocyte.
Discussion
CXCL12 was initially known as a chemotactic factor for T cells and monocytes, and as a growth factor for B cell progenitor cells. In recent years, there has been an increasing number of studies focusing on the pathological characteristics of CXCL12 in the tumor microenvironment (12,13). Particularly, attention has been drawn to the tumor microenvironment suppressing the effectiveness of immune responses by trafficking and retaining these immunosuppressive cells (17). However, further studies are required regarding the role of CXCL12 in OSCC. In the present study, the expression levels of CXCL12 were investigated as a novel prognostic factors in the treatment of OSCC. High expression levels of CXCL12 may contribute to a high recurrence rate and a low 5-year overall survival rate. The results of a previous study are in accordance with the results of the present study. In particular, Clatot et al (18) investigated 71 patients with primary head and neck squamous cell carcinoma, and revealed that CXCL12 expression is significantly associated with metastatic evolution and overall survival.
TILs in the tumor microenvironment may reflect tumor biology and predict prognosis. However, further examination is required to investigate whether lymphocyte infiltration at the primary tumor site represents a beneficial antitumor immune response in the tumor microenvironment, or whether it is implicated as a poor prognostic factor, promoting tumor progression by releasing regulatory cytokines (19,20). CTLs belong to the CD8 population, a major subpopulation which has been suggested to aid in the promotion of immune mediated tumor regression and serve as a response indicator in chemotherapy in a number of types of cancer (7,9). A number of studies have indicated that decreased numbers of nest and stromal CD8+ T cells were associated with lower survival time in patients with OSCC (5,21). The present study reported that the density of CD8+ TILs was not associated with the overall survival or any of the clinicopathological features of OSCC. Therefore, taking the aforementioned into consideration, it is controversial to regard CD8+ TILs as an independent prognostic factor.
The development and function of Tregs depends on the expression of FoxP3, which has been reported as the master regulator (22). Tumor cells have the ability to recruit Tregs to the tumor microenvironment to inhibit antitumor immunity in patients with cancer. It has been reported that a population of FoxP3+ regulatory T cells serves as a predicting factor for survival in colon cancer (22). In the present study, FoxP3+ TILs were significantly associated with tumor recurrence. Furthermore, it was indicated that a high density of FoxP3+ TILs in OSCC tissue is associated with poor survival, however this result was not significant (P=0.083). The number of FoxP3+ TILs tended to be sparse in early stage OSCC and in well-differentiated OSCC. Conversely, in the present study, expression levels of FoxP3+ TILs were higher in advanced stage OSCC and poorly-differentiated OSCC. This finding may indicate that FoxP3+ TILs may serve an important role in suppressing an antitumor immune response in advanced OSCC compared to early OSCC. However, another study revealed that higher peripheral blood levels of this subset were associated with improved survival in patients with oropharyngeal cancer (5). Another study on tumor infiltration suggested that high expression levels of FoxP3 infiltration are associated with an increased survival rate in patients with head and neck cancer (23).
Therefore, the balance between the CD8+ TILs and FoxP3+ TILs is significant. In the present study, the CD8+/FoxP3+ ratio was revealed to be the strongest prognostic indicator and was associated with poor differentiation, advanced stage tumors, tumor recurrence and poor survival rates. A previous study demonstrated that the CD8+/C-C motif chemokine receptor 4+ T-cells ratio was the most significant prognostic factor among all TIL-associated variables (6). Additionally, various types of immune cells can be attracted to the tumor environment via CXCL12 (24,25).
Thus, the present study investigated the associations among CXCL12 expression and FoxP3+ TILs and CD8+ TILs in OSCC. Notably, the present study revealed that FoxP3+ TIL counts and CXCL12 percentage area exhibited a significant correlation. However, no significant correlation was detected for CD8+ TILs and CXCL12 percentage area. In addition, the present study identified that some stroma cells with high CXCL12 expression were surrounded by numerous FoxP3+ TILs, which may support the result of a correlation between FoxP3+ TIL counts and CXCL12 percentage area. Therefore, the present study demonstrated that CXCL12 may contribute to tumor immunosuppression by recruiting FoxP3+ T-cell populations in OSCC. The chemokine CXCL12 is a well-known T-cell chemoattractant that selectively binds its receptors CXCR4 and CXCR7. Activation of Tregs upregulates CXCR4 or CXCR7 expression and drives them to migrate to the tumor microenvironment in a CXCL12-dependent manner (24). A recent study indicated that inhibition of CXCL12 expression may increase the number of tumor-infiltrating lymphocytes and overcome resistance to anti-programmed cell death protein 1 treatment (26). Additionally, CXCL12 and CXCR4 may attract the myeloid-derived suppressor cells into the tumor microenvironment in ovarian cancer (25).
In conclusion, the present study indicated that some clinicopathological parameters, including tumor differentiation, tumor stage and overall survival, were significantly associated with the CD8+/FoxP3+ ratio and CXCL12 expression. Overall, these findings supported the hypothesis that high expression levels of CXCL12 may lead to FoxP3+ T-cells accumulation in the progression of OSCC. However, the underlying mechanism of CXCL12 in recruiting FoxP3+ TILs in OSCC remains unclear. In addition, considering the limited sample size, the subgroup survival analysis of CXCL12 could not be conducted. Therefore, further large and long-term studies are required to validate and supplement the findings of the present study. The present study indicated that CXCL12 is a potential prognostic marker that may assist in the pathological analysis of OSCC. Recently, immunotherapy has become an increasingly important treatment strategy in tumor therapy. Specific blocking of CXCL12 is expected to enhance the effects of immunotherapy for OSCC in the future.
Acknowledgements
The authors would like to thank Professor Xiaoyu Li and Dr Yu Chen from State Key Laboratory of Oral Diseases and pathology department for their technical support and assistance in experiments.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81472532) and Graduate Student's Research and Innovation Fund of Sichuan University (grant no. 2018YJSY106).
Availability of data and materials
The datasets used or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
BZ and CW participated in the design and conducted the experiments, data analysis, and final drafting and writing of the manuscript. ZZ, KY, YL and CL were involved in research design and contributed to the drafting of the manuscript. LL was involved in research design and drafting of the final manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of West China Hospital of Stomatology of Sichuan University. All patients signed informed consent forms prior to the present study.
Patient consent for publication
Informed consent was obtained from all patients for the publication of the present study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.McDowell JD. An overview of epidemiology and common risk factors for oral squamous cell carcinoma. Otolaryngol Clin North Am. 2006;39:277–294. doi: 10.1016/j.otc.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Williams HK. Molecular pathogenesis of oral squamous carcinoma. Mol Pathol. 2000;53:165–172. doi: 10.1136/mp.53.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J, University of Michigan Head and Neck SPORE Program Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015;51:90–95. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:744–752. doi: 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 8.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 9.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 11.Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, van der Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: Which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 12.Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 13.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Xia J, Chen N, Hong Y, Chen X, Tao X, Cheng B, Huang Y. Expressions of CXCL12/CXCR4 in oral premalignant and malignant lesions. Mediators Inflamm. 2012;2012:516395. doi: 10.1155/2012/516395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 17.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clatot F, Picquenot JM, Choussy O, Gouérant S, Moldovan C, Schultheis D, Cornic M, François A, Blot E, Laberge-Le-Couteulx S. Intratumoural level of SDF-1 correlates with survival in head and neck squamous cell carcinoma. Oral Oncol. 2011;47:1062–1068. doi: 10.1016/j.oraloncology.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 20.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, Peterson L, Carey TE, Walline H, Moyer J, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074–1084. doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 23.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 24.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 25.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zboralski D, Hoehlig K, Eulberg D, Frömming A, Vater A. increasing tumor-infiltrating T cells through inhibition of CXCL12 with NOX-A12 synergizes with PD-1 blockade. Cancer Immunol Res. 2017;5:950–956. doi: 10.1158/2326-6066.CIR-16-0303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analysed during the present study are available from the corresponding author on reasonable request.