Table 1.
Ligand and Acid Effects on Asymmetric Hydrophosphinylation of 1aa
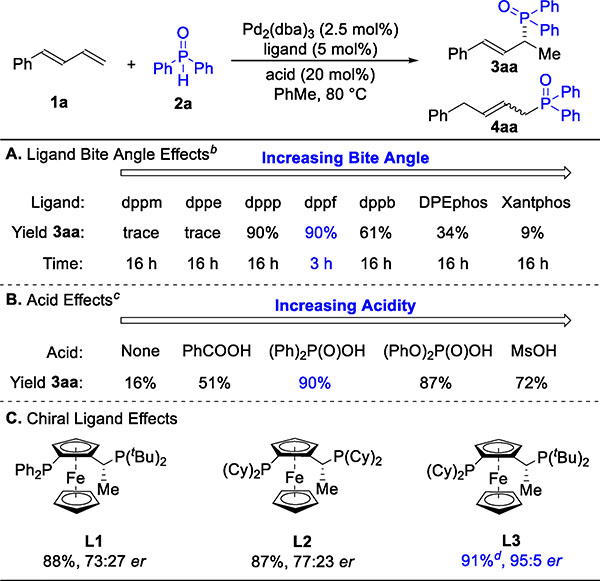 |
Reaction conditions: 1a (0.12 mmol), 2a (0.10 mmol), Pd2(dba)3 (2.5 mol%), ligand (5.0 mol%), acid (20 mol%), toluene (0.40 mL), 3 h (unless otherwise noted). Yield determined by GC-FID analysis of the reaction mixture, which was referenced to 1,3,5-trimethoxybenzene. Regioselectivity ratio (rr) is the ratio of 3aa to 4aa, which is determined by 31P NMR analysis of reaction mixture. Enantioselectivity ratio (er) determined by chiral SFC. See SI for full structure of abbreviations used. Unless otherwise noted, rr is >20:1.
Standard conditions with (Ph)2P(O)OH as acid.
Standard conditions with dppf as ligand.
Isolated yield of 3aa, 3.47 mmol scale, using Pd2(dba)3 (0.50 mol%) and L3 (1.0 mol%) with standard conditions, 18 h.
