Figure 1.
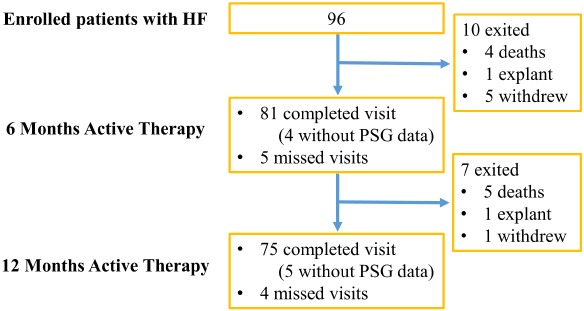
Composition of the pooled study population and follow‐up time points. As pre‐specified in the protocol, patients were implanted and randomized to treatment (therapy activated one month after implant) or control (therapy activated after the 6‐month assessments). This study design allowed for the pooling of 6‐ and 12‐month effectiveness data from the treatment and control groups based on months since therapy activation (baseline for these analyses). Patients in the treatment group accrued 6‐ and 12‐month data at the corresponding visits, whereas the control group accrued 6‐ and 12‐month data at the 12‐ and 18‐month visits due to the delay in initiating therapy. HF, heart failure; PSG, polysomnogram.
