Figure 3.
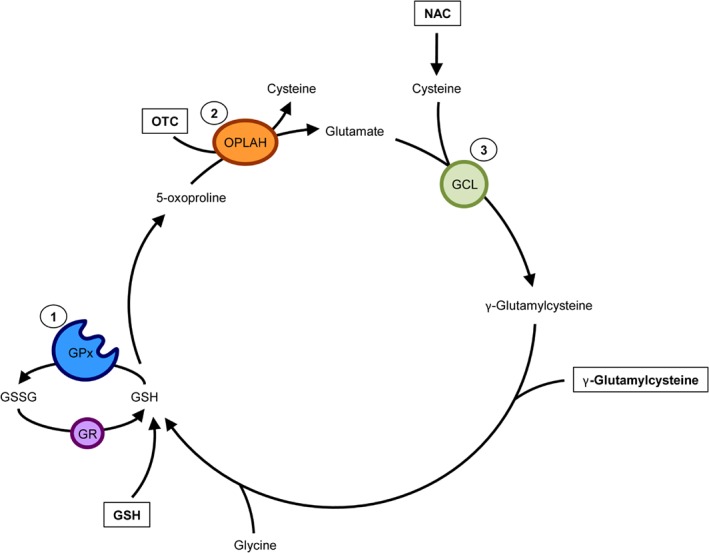
Drug therapies targeting endogenous glutathione (GSH) synthesis. GSH is synthesized from cysteine (the rate‐limiting amino acid), glutamate, and glycine by the γ‐glutamyl cycle. GSH is then utilized by GSH peroxidase (GPx) to reduce oxidative stress, and in the process forming oxidized GSH (GSSG). GSSG is then reduced by action of GSH reductase (GR). Improving the γ‐glutamyl cycle's ability to produce GSH has been characterized as a treatment target in heart failure. N‐acetylcysteine (NAC), γ‐glutamylcysteine, and 2‐oxothiazolidine‐4‐carboxylate (OTC, also known as pro‐cysteine) are compounds which have demonstrated the capacity to increase the endogenous production of GSH. OTC is converted to cysteine, by action of 5‐oxoprolinase (OPLAH), to be used for de novo synthesis of GSH. Similarly, NAC is converted to cysteine intracellularly, and used for GSH synthesis. γ‐Glutamylcysteine is utilized by the γ‐glutamyl cycle to form GSH, by addition of glycine. GCL, glutamate cysteine ligase.
