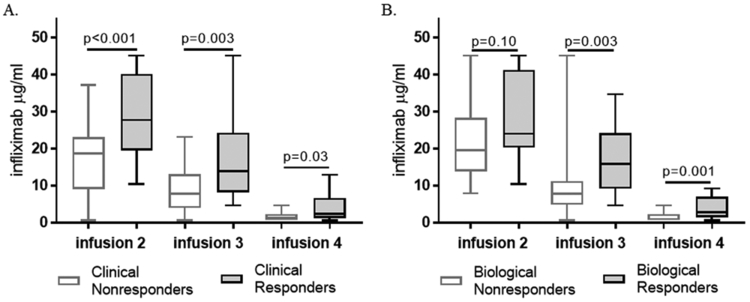
Figure 1. Infliximab trough concentrations for primary and second outcomes.
(A) Clinical response at infusion 4 was determined by improvement in the baseline wPCDAI (delta >17.5) and remaining on infliximab without surgery. (B) Biological response at infusion 4 was defined by >50% improvement from the baseline fecal calprotectin. Drug concentrations at each infusion were compared with the Mann-Whitney test.