Figure 2.
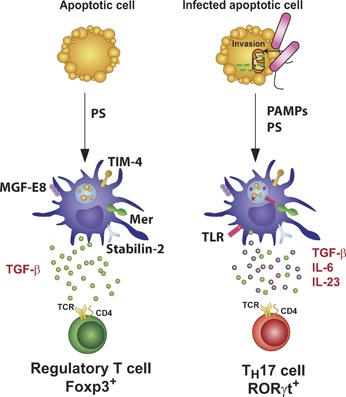
Innate immune recognition of apoptotic cells carrying TLR ligands instructs Th17 cell differentiation. Apoptotic cells express eat‐me signals, including exposure of PS at the outer leaflet of the plasma membrane, which activates PRRs such as TIM‐4, stabilin‐2, BAI‐1, MER, and the αvβ3 integrin expressed on the surface of phagocytes, such as DCs. The receptors MER or the αvβ3 integrin require interaction with bridging molecules such as GAS6 or MFG‐E8, respectively, to recognize PS and initiate phagocytosis. DCs that phagocytose apoptotic cells induce a noninflammatory response, characterized by the production of TGF‐β, resulting in the polarization of Treg cells that secrete anti‐inflammatory cytokines such as TGF‐β and IL‐10. Bacterial invasion of target cells (bacteria are shown here in pink) can induce the release of cytochrome c (green), a characteristic of apoptosis. In this case, DCs are activated in response to apoptotic cell‐derived and TLR‐derived signals. Antigens derived from the apoptotic cell and the microbial pathogen end up in the phagosome, resulting in their presentation on MHC molecules to T cells. This combination of signals induces IL‐6, TGF‐β, and IL‐23, a unique cytokine profile that triggers Th17 differentiation.
